Page 110 - Adsorbents fundamentals and applications
P. 110
ADSORPTION FROM SOLUTION AND EFFECTS OF SURFACE FUNCTIONALITIES 95
stronger than that between thiolane and clay. For adsorption from their aqueous
solutions, on the contrary, Figure 5.11 shows that the adsorption isotherm of
sulfolane is much lower than that of thiolane. The low adsorption of sulfolane
is due to the thermodynamic stability of the sulfolane–water interactions that
are comparable with or stronger than the interactions between sulfolane and
the negatively charged sites of the clay. The opposite holds for thiolane. This
example illustrates the importance of the solute–solvent interaction and also
the complexity of adsorption from liquid solution. The effects of solvent have
been studied for adsorption of “Sudan III” and “Butter Yellow” from different
solvents, and the amounts adsorbed varied over an order of magnitude (Manes
and Hofer, 1969). Another good example for the strong solvent effect is seen
in the adsorption of glycols and sugars with multiple −OH groups from their
aqueous solutions on activated carbon (Chinn and King, 1999). Three adsorbates
are compared: propylene glycol (M.W. = 76, 2 hydroxyls), glycerol (M.W. = 92,
3 hydroxyls), and glucose (M.W. = 180, 5 hydroxyls). The solubility follows the
order of the number of hydroxyl groups, that is, glucose is the most soluble.
Without the solvent, the sorbate–sorbent bond strength follows the order of the
molecular weight. From the aqueous solution, however, the order of the heat
of adsorption is completely reversed (20–30 kJ/mol for propylene glycol and
∼10 kJ/mol for the other two).
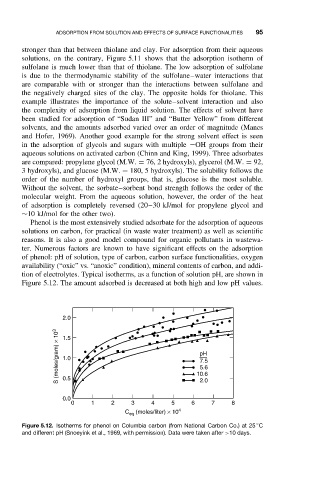
Phenol is the most extensively studied adsorbate for the adsorption of aqueous
solutions on carbon, for practical (in waste water treatment) as well as scientific
reasons. It is also a good model compound for organic pollutants in wastewa-
ter. Numerous factors are known to have significant effects on the adsorption
of phenol: pH of solution, type of carbon, carbon surface functionalities, oxygen
availability (“oxic” vs. “anoxic” condition), mineral contents of carbon, and addi-
tion of electrolytes. Typical isotherms, as a function of solution pH, are shown in
Figure 5.12. The amount adsorbed is decreased at both high and low pH values.
2.0
S (moles/gram) × 10 3 1.5 10.6
pH
1.0
7.5
5.6
0.5
0.0 2.0
0 1 2 3 4 5 6 7 8
C (moles/liter) × 10 4
eq
◦
Figure 5.12. Isotherms for phenol on Columbia carbon (from National Carbon Co.) at 25 C
and different pH (Snoeyink et al., 1969, with permission). Data were taken after >10 days.