Page 115 - Adsorbents fundamentals and applications
P. 115
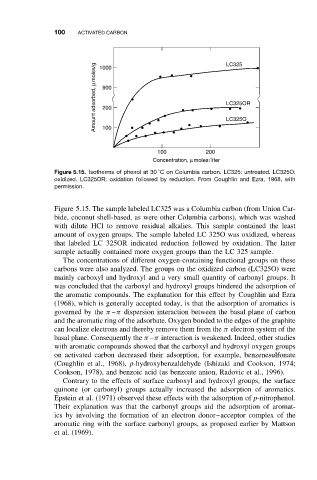
100 ACTIVATED CARBON LC325
Amount adsorbed, µmoles/g 1000 LC325OR
900
200
LC325O
100
100 200
Concentration, µ moles/liter
◦
Figure 5.15. Isotherms of phenol at 30 C on Columbia carbon. LC325: untreated. LC325O:
oxidized. LC325OR: oxidation followed by reduction. From Coughlin and Ezra, 1968, with
permission.
Figure 5.15. The sample labeled LC325 was a Columbia carbon (from Union Car-
bide, coconut shell-based, as were other Columbia carbons), which was washed
with dilute HCl to remove residual alkalies. This sample contained the least
amount of oxygen groups. The sample labeled LC 325O was oxidized, whereas
that labeled LC 325OR indicated reduction followed by oxidation. The latter
sample actually contained more oxygen groups than the LC 325 sample.
The concentrations of different oxygen-containing functional groups on these
carbons were also analyzed. The groups on the oxidized carbon (LC325O) were
mainly carboxyl and hydroxyl and a very small quantity of carbonyl groups. It
was concluded that the carboxyl and hydroxyl groups hindered the adsorption of
the aromatic compounds. The explanation for this effect by Coughlin and Ezra
(1968), which is generally accepted today, is that the adsorption of aromatics is
governed by the π –π dispersion interaction between the basal plane of carbon
and the aromatic ring of the adsorbate. Oxygen bonded to the edges of the graphite
can localize electrons and thereby remove them from the π electron system of the
basal plane. Consequently the π –π interaction is weakened. Indeed, other studies
with aromatic compounds showed that the carboxyl and hydroxyl oxygen groups
on activated carbon decreased their adsorption, for example, benzenesulfonate
(Coughlin et al., 1968), p-hydroxybenzaldehyde (Ishizaki and Cookson, 1974;
Cookson, 1978), and benzoic acid (as benzoate anion, Radovic et al., 1996).
Contrary to the effects of surface carboxyl and hydroxyl groups, the surface
quinone (or carbonyl) groups actually increased the adsorption of aromatics.
Epstein et al. (1971) observed these effects with the adsorption of p-nitrophenol.
Their explanation was that the carbonyl groups aid the adsorption of aromat-
ics by involving the formation of an electron donor–acceptor complex of the
aromatic ring with the surface carbonyl groups, as proposed earlier by Mattson
et al. (1969).