Page 112 - Adsorbents fundamentals and applications
P. 112
ADSORPTION FROM SOLUTION AND EFFECTS OF SURFACE FUNCTIONALITIES 97
2.8 pH 1.8
Reversible adsorption (µ mole/m 2 ) 2.0 unbuffered
2.4
pH 8.0
pH 12.1
1.6
1.2
0.8
0.4
0
0 2 4 6
Phenol concentration (mmole/L)
Irreversible adsorption (µ mole/m 2 ) 2.0 pH 1.8
2.8
2.4
1.6
1.2
pH 8.0
pH 12.1
0.8
unbuffered
0.4
0
0 2 4 6
Phenol concentration (mmole/L)
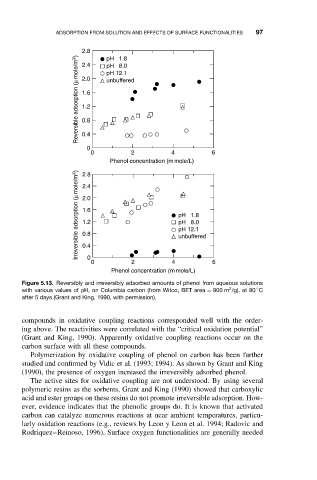
Figure 5.13. Reversibly and irreversibly adsorbed amounts of phenol from aqueous solutions
2
◦
with various values of pH, on Columbia carbon (from Witco, BET area = 900 m /g), at 80 C
after 5 days (Grant and King, 1990, with permission).
compounds in oxidative coupling reactions corresponded well with the order-
ing above. The reactivities were correlated with the “critical oxidation potential”
(Grant and King, 1990). Apparently oxidative coupling reactions occur on the
carbon surface with all these compounds.
Polymerization by oxidative coupling of phenol on carbon has been further
studied and confirmed by Vidic et al. (1993; 1994). As shown by Grant and King
(1990), the presence of oxygen increased the irreversibly adsorbed phenol.
The active sites for oxidative coupling are not understood. By using several
polymeric resins as the sorbents, Grant and King (1990) showed that carboxylic
acid and ester groups on these resins do not promote irreversible adsorption. How-
ever, evidence indicates that the phenolic groups do. It is known that activated
carbon can catalyze numerous reactions at near ambient temperatures, particu-
larly oxidation reactions (e.g., reviews by Leon y Leon et al. 1994; Radovic and
Rodriquez–Reinoso, 1996). Surface oxygen functionalities are generally needed