Page 113 - Adsorbents fundamentals and applications
P. 113
98 ACTIVATED CARBON
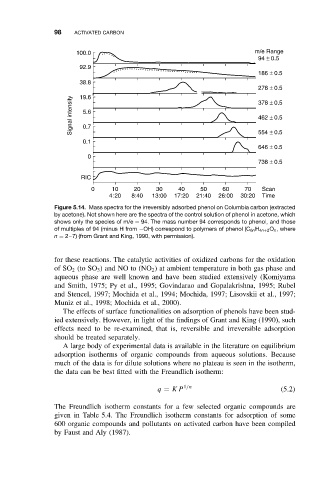
100.0 m/e Range
94 ± 0.5
92.9
186 ± 0.5
38.8
278 ± 0.5
19.6
Signal intensity 5.6 378 ± 0.5
462 ± 0.5
0.7
0.1 554 ± 0.5
646 ± 0.5
0
738 ± 0.5
RIC
0 10 20 30 40 50 60 70 Scan
4:20 8:40 13:00 17:20 21:40 26:00 30:20 Time
Figure 5.14. Mass spectra for the irreversibly adsorbed phenol on Columbia carbon (extracted
by acetone). Not shown here are the spectra of the control solution of phenol in acetone, which
shows only the species of m/e = 94. The mass number 94 corresponds to phenol, and those
of multiples of 94 (minus H from −OH) correspond to polymers of phenol (C 6n H 4n+2 O n ,where
n = 2–7) (from Grant and King, 1990, with permission).
for these reactions. The catalytic activities of oxidized carbons for the oxidation
of SO 2 (to SO 3 )and NO to (NO 2 ) at ambient temperature in both gas phase and
aqueous phase are well known and have been studied extensively (Komiyama
and Smith, 1975; Py et al., 1995; Govindarao and Gopalakrishna, 1995; Rubel
and Stencel, 1997; Mochida et al., 1994; Mochida, 1997; Lisovskii et al., 1997;
Muniz et al., 1998; Mochida et al., 2000).
The effects of surface functionalities on adsorption of phenols have been stud-
ied extensively. However, in light of the findings of Grant and King (1990), such
effects need to be re-examined, that is, reversible and irreversible adsorption
should be treated separately.
A large body of experimental data is available in the literature on equilibrium
adsorption isotherms of organic compounds from aqueous solutions. Because
much of the data is for dilute solutions where no plateau is seen in the isotherm,
the data can be best fitted with the Freundlich isotherm:
q = KP 1/n (5.2)
The Freundlich isotherm constants for a few selected organic compounds are
given in Table 5.4. The Freundlich isotherm constants for adsorption of some
600 organic compounds and pollutants on activated carbon have been compiled
by Faust and Aly (1987).