Page 114 - Adsorbents fundamentals and applications
P. 114
ADSORPTION FROM SOLUTION AND EFFECTS OF SURFACE FUNCTIONALITIES 99
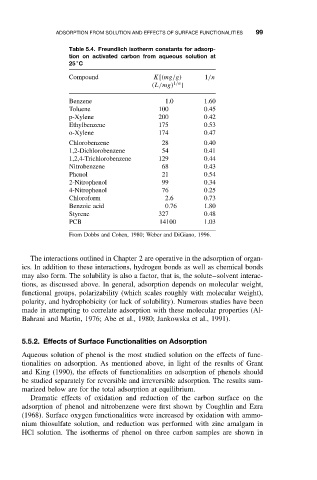
Table 5.4. Freundlich isotherm constants for adsorp-
tion on activated carbon from aqueous solution at
◦
25 C
Compound K[(mg/g) 1/n
(L/mg) 1/n ]
Benzene 1.0 1.60
Toluene 100 0.45
p-Xylene 200 0.42
Ethylbenzene 175 0.53
o-Xylene 174 0.47
Chlorobenzene 28 0.40
1,2-Dichlorobenzene 54 0.41
1,2,4-Trichlorobenzene 129 0.44
Nitrobenzene 68 0.43
Phenol 21 0.54
2-Nitrophenol 99 0.34
4-Nitrophenol 76 0.25
Chloroform 2.6 0.73
Benzoic acid 0.76 1.80
Styrene 327 0.48
PCB 14100 1.03
From Dobbs and Cohen, 1980; Weber and DiGiano, 1996.
The interactions outlined in Chapter 2 are operative in the adsorption of organ-
ics. In addition to these interactions, hydrogen bonds as well as chemical bonds
may also form. The solubility is also a factor, that is, the solute–solvent interac-
tions, as discussed above. In general, adsorption depends on molecular weight,
functional groups, polarizability (which scales roughly with molecular weight),
polarity, and hydrophobicity (or lack of solubility). Numerous studies have been
made in attempting to correlate adsorption with these molecular properties (Al-
Bahrani and Martin, 1976; Abe et al., 1980; Jankowska et al., 1991).
5.5.2. Effects of Surface Functionalities on Adsorption
Aqueous solution of phenol is the most studied solution on the effects of func-
tionalities on adsorption. As mentioned above, in light of the results of Grant
and King (1990), the effects of functionalities on adsorption of phenols should
be studied separately for reversible and irreversible adsorption. The results sum-
marized below are for the total adsorption at equilibrium.
Dramatic effects of oxidation and reduction of the carbon surface on the
adsorption of phenol and nitrobenzene were first shown by Coughlin and Ezra
(1968). Surface oxygen functionalities were increased by oxidation with ammo-
nium thiosulfate solution, and reduction was performed with zinc amalgam in
HCl solution. The isotherms of phenol on three carbon samples are shown in