Page 117 - Adsorbents fundamentals and applications
P. 117
102 ACTIVATED CARBON
It is important to consider the charge of the surface because it determines the
capacity of the carbon for ion exchange. In the aqueous solution of an electrolyte,
an electrical double layer (or diffuse cloud) of dissociated H and OH is formed
−
+
−
on a charged surface. Hydroxide ions (OH ) compose the inner layer of the
electrical double layer on a positively charged surface, whereas protons (H )
+
form the inner layer on a negatively charged surface. Anion exchange occurs on
the positively charged carbon surface via:
−
+
+
−
−
+
C ·· OH + H + A ⇒ C ·· A + H 2 O (5.3)
Cation exchange occurs on the negatively charged surface by:
+
−
+
+
−
−
+
C ·· H + K + A ⇒ C ·· K + H + A − (5.4)
and it is accompanied by acidification of the solution.
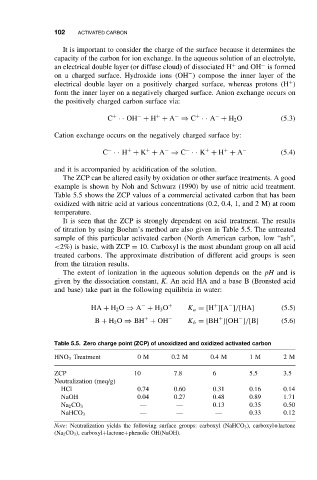
The ZCP can be altered easily by oxidation or other surface treatments. A good
example is shown by Noh and Schwarz (1990) by use of nitric acid treatment.
Table 5.5 shows the ZCP values of a commercial activated carbon that has been
oxidized with nitric acid at various concentrations (0.2, 0.4, 1, and 2 M) at room
temperature.
It is seen that the ZCP is strongly dependent on acid treatment. The results
of titration by using Boehm’s method are also given in Table 5.5. The untreated
sample of this particular activated carbon (North American carbon, low “ash”,
<2%) is basic, with ZCP = 10. Carboxyl is the most abundant group on all acid
treated carbons. The approximate distribution of different acid groups is seen
from the titration results.
The extent of ionization in the aqueous solution depends on the pH and is
given by the dissociation constant, K. An acid HA and a base B (Bronsted acid
and base) take part in the following equilibria in water:
+
−
−
HA + H 2 O ⇒ A + H 3 O + K a = [H ][A ]/[HA] (5.5)
−
+
+
B + H 2 O ⇒ BH + OH − K b = [BH ][OH ]/[B] (5.6)
Table 5.5. Zero charge point (ZCP) of unoxidized and oxidized activated carbon
HNO 3 Treatment 0M 0.2M 0.4M 1 M 2M
ZCP 10 7.8 6 5.5 3.5
Neutralization (meq/g)
HCl 0.74 0.60 0.31 0.16 0.14
NaOH 0.04 0.27 0.48 0.89 1.71
Na 2 CO 3 — — 0.13 0.35 0.50
NaHCO 3 — — — 0.33 0.12
Note: Neutralization yields the following surface groups: carboxyl (NaHCO 3 ), carboxyl+lactone
(Na 2 CO 3 ), carboxyl+lactone+phenolic OH(NaOH).