Page 116 - Adsorbents fundamentals and applications
P. 116
ADSORPTION FROM SOLUTION AND EFFECTS OF SURFACE FUNCTIONALITIES 101
Simple experimental procedures are known for generating the two differ-
ent types of acid oxygen groups on carbon (Cookson, 1978). Surface oxides
◦
developed by chemical treatment and dry oxidation at temperatures <400 C
◦
are mainly the carboxyl and hydroxyl types. At temperatures >400 C, the dry
oxidation treatment yields mainly carbonyl groups (in the form of quinone and
hydroquinone).
The effects of oxygen functionalities on the adsorption of aliphatic compounds
from their aqueous solutions have also been studied (Cookson, 1978; Jankowska
et al., 1991; Radovic, 1996). The adsorption capacities of butyl disulfide and
decane were both decreased by surface oxides (Cookson, 1978). Hence it was con-
cluded that surface oxides hindered adsorption of nonpolar aliphatic compounds.
Radovic et al. (1996) investigated the effects of “nitriding” the surface on
adsorption from solution. Reacting with ammonia at elevated temperatures intro-
◦
duced pyridine functional groups on carbon. Reaction at 200 Cforms amides,
◦
imides, imines, amines, and nitriles; while reaction at 250 C results in bond-
ing of ammonia to the carbon double bonds (Vinke et al., 1994). The effects of
◦
nitriding (at 250 C) were similar to that of oxidation. Nitriding also hindered the
adsorption of benzoate and aliphatic anions, oxalate, and fumarate.
The effects of surface functionalities on adsorption of organic electrolytes,
including weak electrolytes such as phenols, are significantly more complicated
to assess. One needs to consider the surface charge of the carbon as well as the
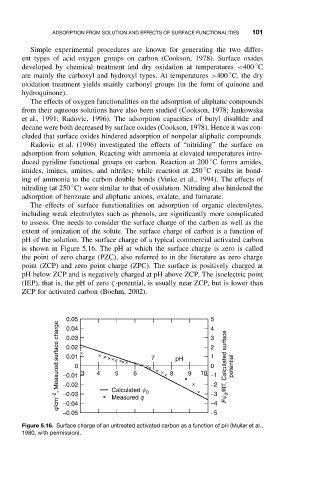
extent of ionization of the solute. The surface charge of carbon is a function of
pH of the solution. The surface charge of a typical commercial activated carbon
is shown in Figure 5.16. The pH at which the surface charge is zero is called
the point of zero charge (PZC), also referred to in the literature as zero charge
point (ZCP) and zero point charge (ZPC). The surface is positively charged at
pH below ZCP and is negatively charged at pH above ZCP. The isoelectric point
(IEP), that is, the pH of zero ζ-potential, is usually near ZCP, but is lower than
ZCP for activated carbon (Boehm, 2002).
0.05 5
q/cm −2 , Measured surface charge −0.01 3 4 5 6 7 8 pH 9 10 2 Fy 0 /RT, Calculated surface potential
0.04
4
0.03
3
0.02
1
0.01
0
0
−1
−2
−0.02
−0.03
Measured q
−4
−0.04
−5
−0.05 Calculated y 0 −3
Figure 5.16. Surface charge of an untreated activated carbon as a function of pH (Muller et al.,
1980, with permission).