Page 148 - Adsorbents fundamentals and applications
P. 148
SILICA GELS: PREPARATION AND GENERAL PROPERTIES 133
The reaction products are high-molecular-weight polysilicates (a sol), which
form a 3-D porous network filled with solvent molecules (a gel). The more recent
development of MCM-41 (to be discussed separately) is a derivative of the sol-
gel route. The pore structure, as well as the surface chemistry, can be tailored
in the sol-gel route. The pH value in the initial stages (Reactions 3–5) is a
main factor in controlling the pore dimensions. Low pH (e.g., by adding HCl)
leads to microporosity, whereas high pH (e.g., by adding ammonium hydroxide)
results in mesoporosity (Brinker and Sherer, 1990). Apparently, pH influences the
size distribution of the globular, primary particles and also how these particles
agglomerate, hence the final pore structure. Sol-gel processing is highly versatile.
It is not limited to silica and is also applicable to many other main group and
transition metal oxides.
The most-used property for silica gel is as a desiccant, that is, adsorbent
for moisture. As mentioned, this quality is due to its relatively weak bonds
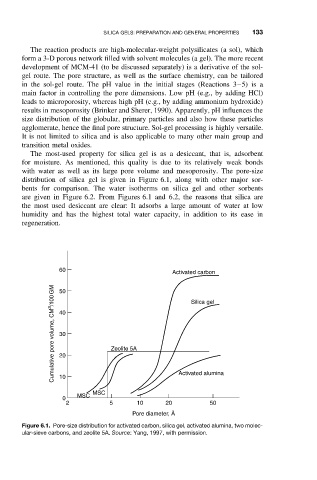
with water as well as its large pore volume and mesoporosity. The pore-size
distribution of silica gel is given in Figure 6.1, along with other major sor-
bents for comparison. The water isotherms on silica gel and other sorbents
are given in Figure 6.2. From Figures 6.1 and 6.2, the reasons that silica are
the most used desiccant are clear: It adsorbs a large amount of water at low
humidity and has the highest total water capacity, in addition to its ease in
regeneration.
60
Activated carbon
Cumulative pore volume, CM 3 /100 GM 40 Zeolite 5A
50
Silica gel
30
20
10
MSC Activated alumina
MSC
0
2 5 10 20 50
Pore diameter, Å
Figure 6.1. Pore-size distribution for activated carbon, silica gel, activated alumina, two molec-
ular-sieve carbons, and zeolite 5A. Source: Yang, 1997, with permission.