Page 152 - Adsorbents fundamentals and applications
P. 152
THE SILANOL NUMBER (OH/NM −1 ) 137
5
4.6
4
a OH OH nm −2 3
2
1
0
200 400 600 800 1000
T (°C)
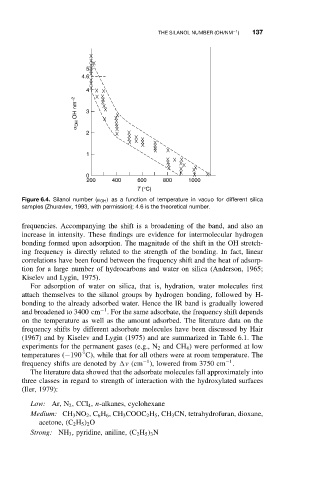
Figure 6.4. Silanol number (α OH ) as a function of temperature in vacuo for different silica
samples (Zhuravlev, 1993, with permission); 4.6 is the theoretical number.
frequencies. Accompanying the shift is a broadening of the band, and also an
increase in intensity. These findings are evidence for intermolecular hydrogen
bonding formed upon adsorption. The magnitude of the shift in the OH stretch-
ing frequency is directly related to the strength of the bonding. In fact, linear
correlations have been found between the frequency shift and the heat of adsorp-
tion for a large number of hydrocarbons and water on silica (Anderson, 1965;
Kiselev and Lygin, 1975).
For adsorption of water on silica, that is, hydration, water molecules first
attach themselves to the silanol groups by hydrogen bonding, followed by H-
bonding to the already adsorbed water. Hence the IR band is gradually lowered
−1
and broadened to 3400 cm . For the same adsorbate, the frequency shift depends
on the temperature as well as the amount adsorbed. The literature data on the
frequency shifts by different adsorbate molecules have been discussed by Hair
(1967) and by Kiselev and Lygin (1975) and are summarized in Table 6.1. The
experiments for the permanent gases (e.g., N 2 and CH 4 ) were performed at low
◦
temperatures (−190 C), while that for all others were at room temperature. The
−1
−1
frequency shifts are denoted by ν (cm ), lowered from 3750 cm .
The literature data showed that the adsorbate molecules fall approximately into
three classes in regard to strength of interaction with the hydroxylated surfaces
(Iler, 1979):
Low: Ar, N 2 , CCl 4 , n-alkanes, cyclohexane
Medium: CH 3 NO 2 ,C 6 H 6 ,CH 3 COOC 2 H 5 ,CH 3 CN, tetrahydrofuran, dioxane,
acetone, (C 2 H 5 ) 2 O
Strong: NH 3 , pyridine, aniline, (C 2 H 5 ) 3 N