Page 149 - Adsorbents fundamentals and applications
P. 149
134 SILICA GEL, MCM, AND ACTIVATED ALUMINA
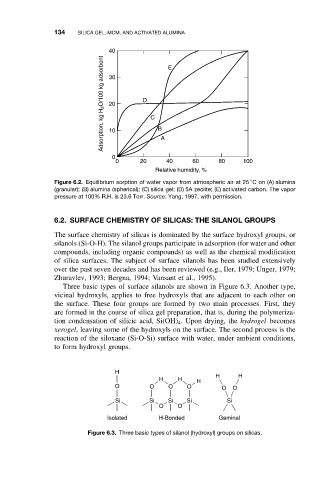
40 E
Adsorption, kg H 2 O/100 kg adsorbent 30 D C B
20
10
0 A
0 20 40 60 80 100
Relative humidity, %
◦
Figure 6.2. Equilibrium sorption of water vapor from atmospheric air at 25 Con(A) alumina
(granular); (B) alumina (spherical); (C) silica gel; (D) 5A zeolite; (E) activated carbon. The vapor
pressure at 100% R.H. is 23.6 Torr. Source: Yang, 1997, with permission.
6.2. SURFACE CHEMISTRY OF SILICAS: THE SILANOL GROUPS
The surface chemistry of silicas is dominated by the surface hydroxyl groups, or
silanols (Si-O-H). The silanol groups participate in adsorption (for water and other
compounds, including organic compounds) as well as the chemical modification
of silica surfaces. The subject of surface silanols has been studied extensively
over the past seven decades and has been reviewed (e.g., Iler, 1979; Unger, 1979;
Zhuravlev, 1993; Bergna, 1994; Vansant et al., 1995).
Three basic types of surface silanols are shown in Figure 6.3. Another type,
vicinal hydroxyls, applies to free hydroxyls that are adjacent to each other on
the surface. These four groups are formed by two main processes. First, they
are formed in the course of silica gel preparation, that is, during the polymeriza-
tion condensation of silicic acid, Si(OH) 4 . Upon drying, the hydrogel becomes
xerogel, leaving some of the hydroxyls on the surface. The second process is the
reaction of the siloxane (Si-O-Si) surface with water, under ambient conditions,
to form hydroxyl groups.
H
H H
H H H
O O O O O O
Si Si Si Si Si
O O
Isolated H-Bonded Geminal
Figure 6.3. Three basic types of silanol (hydroxyl) groups on silicas.