Page 153 - Adsorbents fundamentals and applications
P. 153
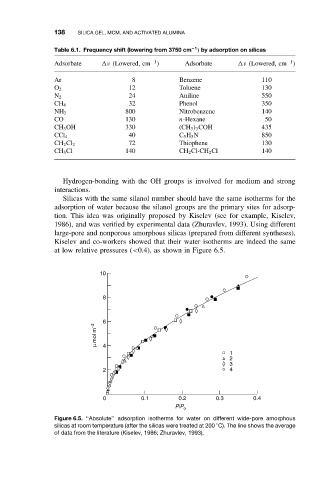
138 SILICA GEL, MCM, AND ACTIVATED ALUMINA
−1
Table 6.1. Frequency shift (lowering from 3750 cm ) by adsorption on silicas
−1
−1
Adsorbate ν (Lowered, cm ) Adsorbate ν (Lowered, cm )
Ar 8 Benzene 110
O 2 12 Toluene 130
N 2 24 Aniline 550
CH 4 32 Phenol 350
800 Nitrobenzene 140
NH 3
CO 130 n-Hexane 50
CH 3 OH 330 (CH 3 ) 3 COH 435
40 C 5 H 5 N 850
CCl 4
72 Thiophene 130
CH 2 Cl 2
CH 3 Cl 140 CH 2 Cl-CH 2 Cl 140
Hydrogen-bonding with the OH groups is involved for medium and strong
interactions.
Silicas with the same silanol number should have the same isotherms for the
adsorption of water because the silanol groups are the primary sites for adsorp-
tion. This idea was originally proposed by Kiselev (see for example, Kiselev,
1986), and was verified by experimental data (Zhuravlev, 1993). Using different
large-pore and nonporous amorphous silicas (prepared from different syntheses),
Kiselev and co-workers showed that their water isotherms are indeed the same
at low relative pressures (<0.4), as shown in Figure 6.5.
10
8
6
µ mol m −2
4
1
2
3
2 4
0 0.1 0.2 0.3 0.4
P/P o
Figure 6.5. ‘‘Absolute’’ adsorption isotherms for water on different wide-pore amorphous
◦
silicas at room temperature (after the silicas were treated at 200 C). The line shows the average
of data from the literature (Kiselev, 1986; Zhuravlev, 1993).