Page 168 - Adsorbents fundamentals and applications
P. 168
ACTIVATED ALUMINA AS SPECIAL SORBENTS 153
1.00
Freundlich model:
K = 0.1679
1/n = 0.452
q o (mg As[V]/g AA)
Batch
Freundlich
0.10
0.1 1 10 100
Co (µg/L)
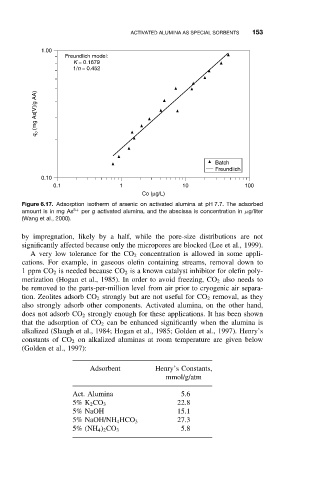
Figure 6.17. Adsorption isotherm of arsenic on activated alumina at pH 7.7. The adsorbed
amount is in mg As 5+ per g activated alumina, and the abscissa is concentration in µg/liter
(Wang et al., 2000).
by impregnation, likely by a half, while the pore-size distributions are not
significantly affected because only the micropores are blocked (Lee et al., 1999).
A very low tolerance for the CO 2 concentration is allowed in some appli-
cations. For example, in gaseous olefin containing streams, removal down to
1 ppm CO 2 is needed because CO 2 is a known catalyst inhibitor for olefin poly-
merization (Hogan et al., 1985). In order to avoid freezing, CO 2 also needs to
be removed to the parts-per-million level from air prior to cryogenic air separa-
tion. Zeolites adsorb CO 2 strongly but are not useful for CO 2 removal, as they
also strongly adsorb other components. Activated alumina, on the other hand,
does not adsorb CO 2 strongly enough for these applications. It has been shown
that the adsorption of CO 2 can be enhanced significantly when the alumina is
alkalized (Slaugh et al., 1984; Hogan et al., 1985; Golden et al., 1997). Henry’s
constants of CO 2 on alkalized aluminas at room temperature are given below
(Golden et al., 1997):
Adsorbent Henry’s Constants,
mmol/g/atm
Act. Alumina 5.6
22.8
5% K 2 CO 3
5% NaOH 15.1
27.3
5% NaOH/NH 4 HCO 3
5.8
5% (NH 4 ) 2 CO 3