Page 163 - Adsorbents fundamentals and applications
P. 163
148 SILICA GEL, MCM, AND ACTIVATED ALUMINA
0.04
30
0.035
0.03 25
Pore volume (cm 3 per g) 0.015 Change in volume adsorbed (cm 3 per g) 15
20
0.025
0.02
10
5
0.0 0.1 0.2 0.3 0.4 0.5 0.6 0.7
0.01
P/P 0
0.005
0
0 20 40 60 80 100
Pore diameter (Å)
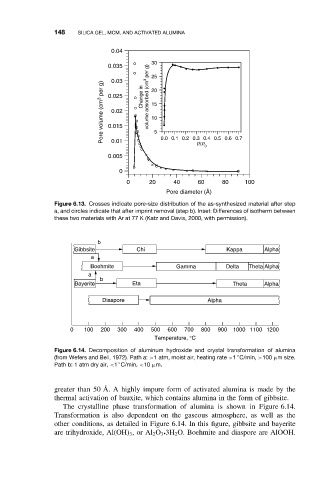
Figure 6.13. Crosses indicate pore-size distribution of the as-synthesized material after step
a, and circles indicate that after imprint removal (step b). Inset: Differences of isotherm between
these two materials with Ar at 77 K (Katz and Davis, 2000, with permission).
b
Gibbsite Chi Kappa Alpha
a
Boehmite Gamma Delta Theta Alpha
a
b
Bayerite Eta Theta Alpha
Diaspore Alpha
0 100 200 300 400 500 600 700 800 900 1000 1100 1200
Temperature, °C
Figure 6.14. Decomposition of aluminum hydroxide and crystal transformation of alumina
◦
(from Wefers and Bell, 1972). Path a: >1 atm, moist air, heating rate >1 C/min, >100 µmsize.
◦
Path b: 1 atm dry air, <1 C/min, <10 µm.
greater than 50 ˚ A. A highly impure form of activated alumina is made by the
thermal activation of bauxite, which contains alumina in the form of gibbsite.
The crystalline phase transformation of alumina is shown in Figure 6.14.
Transformation is also dependent on the gaseous atmosphere, as well as the
other conditions, as detailed in Figure 6.14. In this figure, gibbsite and bayerite
•3H 2 O. Boehmite and diaspore are AlOOH.
are trihydroxide, Al(OH) 3 ,or Al 2 O 3