Page 164 - Adsorbents fundamentals and applications
P. 164
ACTIVATED ALUMINA 149
These natural forms of hydroxides have well-defined crystal structures (Wefers
and Bell, 1972). Upon heat-treatment, they transform into different forms of alu-
mina, shown by Figure 6.14. Among the aluminas, the γ -alumina is the most
commonly used form for both adsorption and catalysis.
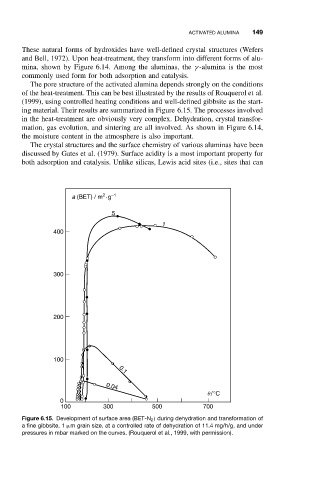
The pore structure of the activated alumina depends strongly on the conditions
of the heat-treatment. This can be best illustrated by the results of Rouquerol et al.
(1999), using controlled heating conditions and well-defined gibbsite as the start-
ing material. Their results are summarized in Figure 6.15. The processes involved
in the heat-treatment are obviously very complex. Dehydration, crystal transfor-
mation, gas evolution, and sintering are all involved. As shown in Figure 6.14,
the moisture content in the atmosphere is also important.
The crystal structures and the surface chemistry of various aluminas have been
discussed by Gates et al. (1979). Surface acidity is a most important property for
both adsorption and catalysis. Unlike silicas, Lewis acid sites (i.e., sites that can
2
a (BET) / m ·g −1
5
1
400
300
200
100 0.1
0.04
q/°C
0
100 300 500 700
Figure 6.15. Development of surface area (BET-N 2 ) during dehydration and transformation of
a fine gibbsite, 1 µm grain size, at a controlled rate of dehydration of 11.4 mg/h/g, and under
pressures in mbar marked on the curves. (Rouquerol et al., 1999, with permission).