Page 189 - Adsorbents fundamentals and applications
P. 189
174 ZEOLITES AND MOLECULAR SIEVES
O O
Cation
O T O
T
O
O O
O O
T T
O O
O
O O
O O
T T
O
O
Ag
16 B
B 9
O
O
15 B O O
Si Al O B 8
O
O
14 B B 7
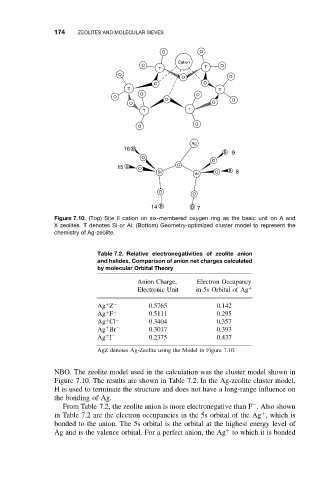
Figure 7.10. (Top) Site II cation on six-membered oxygen ring as the basic unit on A and
X zeolites. T denotes Si or Al. (Bottom) Geometry-optimized cluster model to represent the
chemistry of Ag-zeolite.
Table 7.2. Relative electronegativities of zeolite anion
and halides. Comparison of anion net charges calculated
by molecular Orbital Theory
Anion Charge, Electron Occupancy
Electronic Unit in 5s Orbital of Ag +
+ −
Ag Z 0.5765 0.142
+ −
Ag F 0.5111 0.295
+
Ag Cl − 0.3404 0.357
+
Ag Br − 0.3017 0.393
+ −
Ag I 0.2375 0.437
AgZ denotes Ag-Zeolite using the Model in Figure 7.10.
NBO. The zeolite model used in the calculation was the cluster model shown in
Figure 7.10. The results are shown in Table 7.2. In the Ag-zeolite cluster model,
H is used to terminate the structure and does not have a long-range influence on
the bonding of Ag.
−
From Table 7.2, the zeolite anion is more electronegative than F . Also shown
+
in Table 7.2 are the electron occupancies in the 5s orbital of the Ag ,which is
bonded to the anion. The 5s orbital is the orbital at the highest energy level of
Ag and is the valence orbital. For a perfect anion, the Ag to which it is bonded
+