Page 192 - Adsorbents fundamentals and applications
P. 192
INTERACTIONS OF ADSORBATE WITH CATIONS 177
II
3 1 4 II
4
III III
2 4
1
I
I
III
II
I
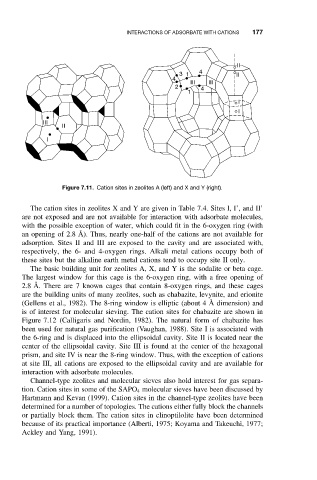
Figure 7.11. Cation sites in zeolites A (left) and X and Y (right).
The cation sites in zeolites X and Y are given in Table 7.4. Sites I, I’, and II’
are not exposed and are not available for interaction with adsorbate molecules,
with the possible exception of water, which could fit in the 6-oxygen ring (with
an opening of 2.8 ˚ A). Thus, nearly one-half of the cations are not available for
adsorption. Sites II and III are exposed to the cavity and are associated with,
respectively, the 6- and 4-oxygen rings. Alkali metal cations occupy both of
these sites but the alkaline earth metal cations tend to occupy site II only.
The basic building unit for zeolites A, X, and Y is the sodalite or beta cage.
The largest window for this cage is the 6-oxygen ring, with a free opening of
2.8 ˚ A. There are 7 known cages that contain 8-oxygen rings, and these cages
are the building units of many zeolites, such as chabazite, levynite, and erionite
(Gellens et al., 1982). The 8-ring window is elliptic (about 4 ˚ A dimension) and
is of interest for molecular sieving. The cation sites for chabazite are shown in
Figure 7.12 (Calligaris and Nordin, 1982). The natural form of chabazite has
been used for natural gas purification (Vaughan, 1988). Site I is associated with
the 6-ring and is displaced into the ellipsoidal cavity. Site II is located near the
center of the ellipsoidal cavity. Site III is found at the center of the hexagonal
prism, and site IV is near the 8-ring window. Thus, with the exception of cations
at site III, all cations are exposed to the ellipsoidal cavity and are available for
interaction with adsorbate molecules.
Channel-type zeolites and molecular sieves also hold interest for gas separa-
tion. Cation sites in some of the SAPO 4 molecular sieves have been discussed by
Hartmann and Kevan (1999). Cation sites in the channel-type zeolites have been
determined for a number of topologies. The cations either fully block the channels
or partially block them. The cation sites in clinoptilolite have been determined
because of its practical importance (Alberti, 1975; Koyama and Takeuchi, 1977;
Ackley and Yang, 1991).