Page 196 - Adsorbents fundamentals and applications
P. 196
INTERACTIONS OF ADSORBATE WITH CATIONS 181
35
N 2
30
O 2
25
Capacity (cc/g) 20
15
10
5
0
0 20 40 60 80 100
Number of Li ions/unit cell
◦
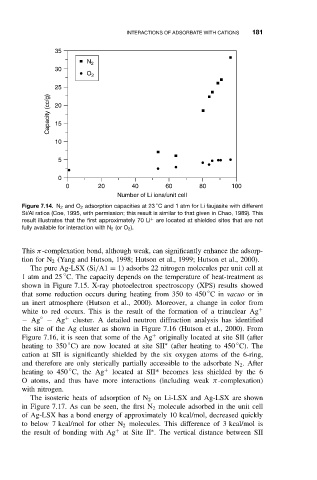
Figure 7.14. N 2 and O 2 adsorption capacities at 23 C and 1 atm for Li faujasite with different
Si/Al ratios (Coe, 1995, with permission; this result is similar to that given in Chao, 1989). This
+
result illustrates that the first approximately 70 Li are located at shielded sites that are not
fully available for interaction with N 2 (or O 2 ).
This π-complexation bond, although weak, can significantly enhance the adsorp-
tion for N 2 (Yang and Hutson, 1998; Hutson et al., 1999; Hutson et al., 2000).
The pure Ag-LSX (Si/A1 = 1) adsorbs 22 nitrogen molecules per unit cell at
◦
1atm and 25 C. The capacity depends on the temperature of heat-treatment as
shown in Figure 7.15. X-ray photoelectron spectroscopy (XPS) results showed
◦
that some reduction occurs during heating from 350 to 450 Cin vacuo or in
an inert atmosphere (Hutson et al., 2000). Moreover, a change in color from
white to red occurs. This is the result of the formation of a trinuclear Ag +
◦
− Ag − Ag + cluster. A detailed neutron diffraction analysis has identified
the site of the Ag cluster as shown in Figure 7.16 (Hutson et al., 2000). From
+
Figure 7.16, it is seen that some of the Ag originally located at site SII (after
◦
◦
∗
heating to 350 C) are now located at site SII (after heating to 450 C). The
cation at SII is significantly shielded by the six oxygen atoms of the 6-ring,
and therefore are only sterically partially accessible to the adsorbate N 2 .After
◦
heating to 450 C, the Ag + located at SII* becomes less shielded by the 6
O atoms, and thus have more interactions (including weak π-complexation)
with nitrogen.
The isosteric heats of adsorption of N 2 on Li-LSX and Ag-LSX are shown
in Figure 7.17. As can be seen, the first N 2 molecule adsorbed in the unit cell
of Ag-LSX has a bond energy of approximately 10 kcal/mol, decreased quickly
to below 7 kcal/mol for other N 2 molecules. This difference of 3 kcal/mol is
the result of bonding with Ag + at Site II . The vertical distance between SII
∗