Page 198 - Adsorbents fundamentals and applications
P. 198
INTERACTIONS OF ADSORBATE WITH CATIONS 183
SII*
SII
SII'
SI'
SI'*
SI
SI'
(a) (b)
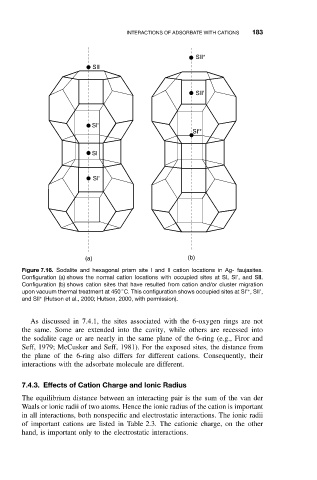
Figure 7.16. Sodalite and hexagonal prism site I and II cation locations in Ag- faujasites.
Configuration (a) shows the normal cation locations with occupied sites at SI, SI’, and SII.
Configuration (b) shows cation sites that have resulted from cation and/or cluster migration
◦
upon vacuum thermal treatment at 450 C. This configuration shows occupied sites at SI’ , SII’,
∗
∗
and SII (Hutson et al., 2000; Hutson, 2000, with permission).
As discussed in 7.4.1, the sites associated with the 6-oxygen rings are not
the same. Some are extended into the cavity, while others are recessed into
the sodalite cage or are nearly in the same plane of the 6-ring (e.g., Firor and
Seff, 1979; McCusker and Seff, 1981). For the exposed sites, the distance from
the plane of the 6-ring also differs for different cations. Consequently, their
interactions with the adsorbate molecule are different.
7.4.3. Effects of Cation Charge and Ionic Radius
The equilibrium distance between an interacting pair is the sum of the van der
Waals or ionic radii of two atoms. Hence the ionic radius of the cation is important
in all interactions, both nonspecific and electrostatic interactions. The ionic radii
of important cations are listed in Table 2.3. The cationic charge, on the other
hand, is important only to the electrostatic interactions.