Page 201 - Adsorbents fundamentals and applications
P. 201
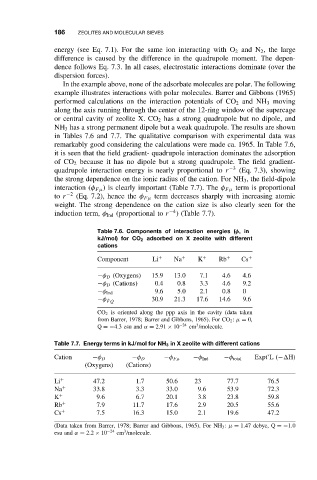
186 ZEOLITES AND MOLECULAR SIEVES
energy (see Eq. 7.1). For the same ion interacting with O 2 and N 2 ,the large
difference is caused by the difference in the quadrupole moment. The depen-
dence follows Eq. 7.3. In all cases, electrostatic interactions dominate (over the
dispersion forces).
In the example above, none of the adsorbate molecules are polar. The following
example illustrates interactions with polar molecules. Barrer and Gibbons (1965)
performed calculations on the interaction potentials of CO 2 and NH 3 moving
along the axis running through the center of the 12-ring window of the supercage
or central cavity of zeolite X. CO 2 has a strong quadrupole but no dipole, and
NH 3 has a strong permanent dipole but a weak quadrupole. The results are shown
in Tables 7.6 and 7.7. The qualitative comparison with experimental data was
remarkably good considering the calculations were made ca. 1965. In Table 7.6,
it is seen that the field gradient- quadrupole interaction dominates the adsorption
of CO 2 because it has no dipole but a strong quadrupole. The field gradient-
quadrupole interaction energy is nearly proportional to r −3 (Eq. 7.3), showing
the strong dependence on the ionic radius of the cation. For NH 3 , the field-dipole
interaction (φ Fµ ) is clearly important (Table 7.7). The φ Fµ term is proportional
to r −2 (Eq. 7.2), hence the φ Fµ term decreases sharply with increasing atomic
weight. The strong dependence on the cation size is also clearly seen for the
induction term, φ Ind (proportional to r −4 )(Table7.7).
Table 7.6. Components of interaction energies (φ,in
kJ/mol) for CO 2 adsorbed on X zeolite with different
cations
Component Li + Na + K + Rb + Cs +
−φ D (Oxygens) 15.9 13.0 7.1 4.6 4.6
−φ D (Cations) 0.4 0.8 3.3 4.6 9.2
−φ Ind 9.6 5.0 2.1 0.8 0
30.9 21.3 17.6 14.6 9.6
−φ ˙ FQ
CO 2 is oriented along the ppp axis in the cavity (data taken
from Barrer, 1978; Barrer and Gibbons, 1965). For CO 2 : µ = 0,
3
Q =−4.3esu and α = 2.91 × 10 −24 cm /molecule.
Table 7.7. Energy terms in kJ/mol for NH 3 in X zeolite with different cations
Cation −φ D −φ D −φ Fµ −φ Ind −φ total Expt’L (− H)
(Oxygens) (Cations)
Li + 47.2 1.7 50.6 23 77.7 76.5
Na + 33.8 3.3 33.0 9.6 53.9 72.3
K + 9.6 6.7 20.1 3.8 23.8 59.8
Rb + 7.9 11.7 17.6 2.9 20.5 55.6
Cs + 7.5 16.3 15.0 2.1 19.6 47.2
(Data taken from Barrer, 1978; Barrer and Gibbons, 1965). For NH 3 : µ = 1.47 debye, Q =−1.0
3
esu and α = 2.2 × 10 −24 cm /molecule.