Page 200 - Adsorbents fundamentals and applications
P. 200
INTERACTIONS OF ADSORBATE WITH CATIONS 185
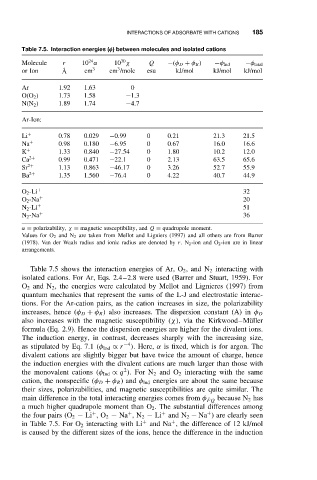
Table 7.5. Interaction energies (φ) between molecules and isolated cations
30
24
Molecule r 10 α 10 χ Q −(φ D + φ R ) −φ Ind −φ total
3
or Ion ˚ A cm 3 cm /molc esu kJ/mol kJ/mol kJ/mol
Ar 1.92 1.63 0
O(O 2 ) 1.73 1.58 −1.3
N(N 2 ) 1.89 1.74 −4.7
Ar-Ion:
Li + 0.78 0.029 −0.99 0 0.21 21.3 21.5
Na + 0.98 0.180 −6.95 0 0.67 16.0 16.6
K + 1.33 0.840 −27.54 0 1.80 10.2 12.0
Ca 2+ 0.99 0.471 −22.1 0 2.13 63.5 65.6
Sr 2+ 1.13 0.863 −46.17 0 3.26 52.7 55.9
Ba 2+ 1.35 1.560 −76.4 0 4.22 40.7 44.9
O 2 -Li + 32
O 2 -Na + 20
N 2 -Li + 51
N 2 -Na + 36
α = polarizability, χ = magnetic susceptibility, and Q = quadrupole moment.
Values for O 2 and N 2 are taken from Mellot and Ligniers (1997) and all others are from Barrer
(1978). Van der Waals radius and ionic radius are denoted by r.N 2 -ion and O 2 -ion are in linear
arrangements.
Table 7.5 shows the interaction energies of Ar, O 2 ,and N 2 interacting with
isolated cations. For Ar, Eqs. 2.4–2.8 were used (Barrer and Stuart, 1959). For
O 2 and N 2 , the energies were calculated by Mellot and Lignieres (1997) from
quantum mechanics that represent the sums of the L-J and electrostatic interac-
tions. For the Ar-cation pairs, as the cation increases in size, the polarizability
increases, hence (φ D + φ R ) also increases. The dispersion constant (A) in φ D
also increases with the magnetic susceptibility (χ), via the Kirkwood–M¨ uller
formula (Eq. 2.9). Hence the dispersion energies are higher for the divalent ions.
The induction energy, in contrast, decreases sharply with the increasing size,
as stipulated by Eq. 7.1 (φ Ind ∝ r −4 ). Here, α is fixed, which is for argon. The
divalent cations are slightly bigger but have twice the amount of charge, hence
the induction energies with the divalent cations are much larger than those with
2
the monovalent cations (φ Ind ∝ q ).For N 2 and O 2 interacting with the same
cation, the nonspecific (φ D + φ R )and φ Ind energies are about the same because
their sizes, polarizabilities, and magnetic susceptibilities are quite similar. The
because N 2 has
main difference in the total interacting energies comes from φ ˙ FQ
a much higher quadrupole moment than O 2 . The substantial differences among
+
+
+
the four pairs (O 2 − Li ,O 2 − Na ,N 2 − Li and N 2 − Na ) are clearly seen
+
+
+
in Table 7.5. For O 2 interacting with Li and Na , the difference of 12 kJ/mol
is caused by the different sizes of the ions, hence the difference in the induction