Page 186 - Adsorbents fundamentals and applications
P. 186
ZEOLITES AND MOLECULAR SIEVES: SYNTHESIS AND MOLECULAR SIEVING PROPERTIES 171
1.1
1 CO2 Adsorption
CH4 Adsorption
0.9 N2 Adsorption
O2 Adsorption
0.8
0.7
q (m mol/g) 0.6
0.5
0.4
0.3
0.2
0.1
0 0 0.2 0.4 0.6 0.8 1
Pressure (atm)
◦
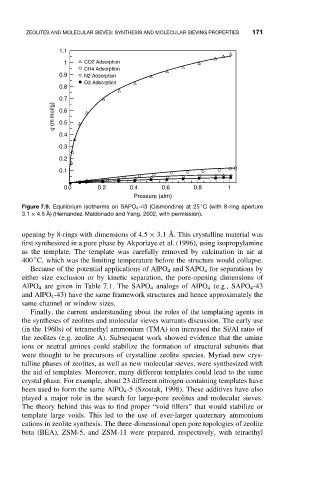
Figure 7.9. Equilibrium isotherms on SAPO 4 -43 (Gismondine) at 25 C (with 8-ring aperture
3.1 × 4.5 ˚ A) (Hernandez-Maldonado and Yang, 2002, with permission).
opening by 8-rings with dimensions of 4.5 × 3.1 ˚ A. This crystalline material was
first synthesized in a pure phase by Akporiaye et al. (1996), using isopropylamine
as the template. The template was carefully removed by calcination in air at
◦
400 C, which was the limiting temperature before the structure would collapse.
Because of the potential applications of AlPO 4 and SAPO 4 for separations by
either size exclusion or by kinetic separation, the pore-opening dimensions of
AlPO 4 are given in Table 7.1. The SAPO 4 analogs of AlPO 4 (e.g., SAPO 4 -43
and AlPO 4 -43) have the same framework structures and hence approximately the
same channel or window sizes.
Finally, the current understanding about the roles of the templating agents in
the syntheses of zeolites and molecular sieves warrants discussion. The early use
(in the 1960s) of tetramethyl ammonium (TMA) ion increased the Si/Al ratio of
the zeolites (e.g. zeolite A). Subsequent work showed evidence that the amine
ions or neutral amines could stabilize the formation of structural subunits that
were thought to be precursors of crystalline zeolite species. Myriad new crys-
talline phases of zeolites, as well as new molecular sieves, were synthesized with
the aid of templates. Moreover, many different templates could lead to the same
crystal phase. For example, about 23 different nitrogen-containing templates have
been used to form the same AlPO 4 -5 (Szostak, 1998). These additives have also
played a major role in the search for large-pore zeolites and molecular sieves.
The theory behind this was to find proper “void fillers” that would stabilize or
template large voids. This led to the use of ever-larger quaternary ammonium
cations in zeolite synthesis. The three-dimensional open pore topologies of zeolite
beta (BEA), ZSM-5, and ZSM-11 were prepared, respectively, with tetraethyl