Page 183 - Adsorbents fundamentals and applications
P. 183
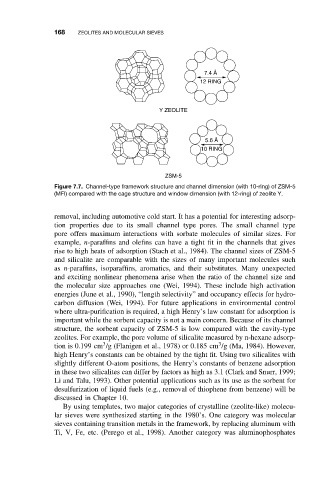
168 ZEOLITES AND MOLECULAR SIEVES
7.4 Å
12 RING
Y ZEOLITE
5.6 Å
10 RING
ZSM-5
Figure 7.7. Channel-type framework structure and channel dimension (with 10-ring) of ZSM-5
(MFI) compared with the cage structure and window dimension (with 12-ring) of zeolite Y.
removal, including automotive cold start. It has a potential for interesting adsorp-
tion properties due to its small channel type pores. The small channel type
pore offers maximum interactions with sorbate molecules of similar sizes. For
example, n-paraffins and olefins can have a tight fit in the channels that gives
rise to high heats of adsorption (Stach et al., 1984). The channel sizes of ZSM-5
and silicalite are comparable with the sizes of many important molecules such
as n-paraffins, isoparaffins, aromatics, and their substitutes. Many unexpected
and exciting nonlinear phenomena arise when the ratio of the channel size and
the molecular size approaches one (Wei, 1994). These include high activation
energies (June et al., 1990), “length selectivity” and occupancy effects for hydro-
carbon diffusion (Wei, 1994). For future applications in environmental control
where ultra-purification is required, a high Henry’s law constant for adsorption is
important while the sorbent capacity is not a main concern. Because of its channel
structure, the sorbent capacity of ZSM-5 is low compared with the cavity-type
zeolites. For example, the pore volume of silicalite measured by n-hexane adsorp-
3
3
tion is 0.199 cm /g (Flanigen et al., 1978) or 0.185 cm /g (Ma, 1984). However,
high Henry’s constants can be obtained by the tight fit. Using two silicalites with
slightly different O-atom positions, the Henry’s constants of benzene adsorption
in these two silicalites can differ by factors as high as 3.1 (Clark and Snurr, 1999;
Li and Talu, 1993). Other potential applications such as its use as the sorbent for
desulfurization of liquid fuels (e.g., removal of thiophene from benzene) will be
discussed in Chapter 10.
By using templates, two major categories of crystalline (zeolite-like) molecu-
lar sieves were synthesized starting in the 1980’s. One category was molecular
sieves containing transition metals in the framework, by replacing aluminum with
Ti, V, Fe, etc. (Perego et al., 1998). Another category was aluminophosphates