Page 66 - Adsorption Technology & Design, Elsevier (1998)
P. 66
Fundamentals of adsorption equilibria 63
where
A~j = (Ai~ A~ )ll(i+]) (3.52)
A condition which must be met is (ial + ja2) <- v, where ttl and a2 are the
effective volumes of the respective adsorbate molecules, v is the cage
volume, and i and j are the number of molecules of species 1 and 2,
respectively, in a cage.
The model outlined has been successful for predicting the mixed
adsorption of hydrocarbons adsorbed on zeolites from single component
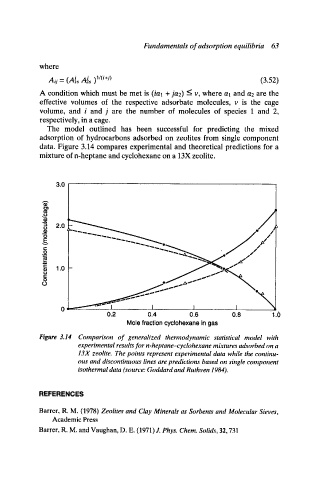
data. Figure 3.14 compares experimental and theoretical predictions for a
mixture of n-heptane and cyclohexane on a 13X zeolite.
3.0
2.0
u
v
t-
O
,,=...
E
r
1.0
u
0
(.3
I I ...... 1 I xa
o.a 0.4 0.6 o.8 1_0
Mole fraction cyclohexane in gas
Figure 3.14 Comparison of generalized thermodynamic statistical model with
experimental results for n-heptane-cyclohexane mixtures adsorbed on a
13X zeolite. The points represent experimental data while the continu-
ous and discontinuous lines are predictions based on single component
isothermal data (source: Goddard and Ruthven 1984).
REFERENCES
Barrer, R. M. (1978) Zeolites and Clay Minerals as Sorbents and Molecular Sieves,
Academic Press
Barrer, R. M. and Vaughan, D. E. (1971) J. Phys. Chem. Solids, 32, 731