Page 64 - Adsorption Technology & Design, Elsevier (1998)
P. 64
Fundamentals of adsorption equilibria 6I
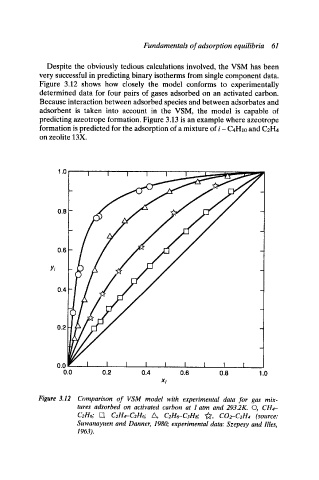
Despite the obviously tedious calculations involved, the VSM has been
very successful in predicting binary isotherms from single component data.
Figure 3.12 shows how closely the model conforms to experimentally
determined data for four pairs of gases adsorbed on an activated carbon.
Because interaction between adsorbed species and between adsorbates and
adsorbent is taken into account in the VSM, the model is capable of
predicting azeotrope formation. Figure 3.13 is an example where azeotrope
formation is predicted for the adsorption of a mixture of i - C4Hl0 and C2H4
on zeolite 13X.
1.0
0.8
0.6
0.4
0.2
O.OIr I . I ...... I I I . I I I I I
0.0 0.2 0.4 0.6 0.8 1.0
xi
Figure 3.12 Comparison of VSM model with experimental data for gas mix-
tures adsorbed on activated carbon at 1 atm and 293.2K. O, CH4-
C2H6; [2, C2H4-C2H6; A, C2H6-C3Hs; "~, C02--C2H4 (source:
Suwanayuen and Danner, 1980; experimental data: Szepesy and flies,
1963).