Page 61 - Adsorption Technology & Design, Elsevier (1998)
P. 61
58 Fundamentals of adsorption equilibria
1.0
0.8
r
r
"t3
(D
..O
O
0.6
"1o
r
r
i
t"q
O
c-
0.4
O
.m
t~
|
o
0.2
!
I I I I
0 0.2 0.4 0.6 0.8 1.0
Mole fraction C2 H6in gas phase
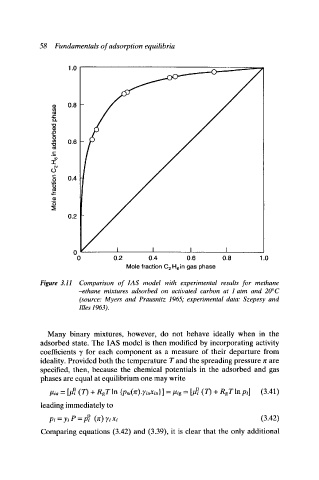
Figure 3.11 Comparison of IAS model with experimental results for methane
-ethane mixtures adsorbed on activated carbon at 1 atm and 20~
(source: Myers and Prausnitz 1965, experimental data: Szepesy and
flies 1963).
Many binary mixtures, however, do not behave ideally when in the
adsorbed state. The IAS model is then modified by incorporating activity
coefficients 7 for each component as a measure of their departure from
ideality. Provided both the temperature T and the spreading pressure zr are
specified, then, because the chemical potentials in the adsorbed and gas
phases are equal at equilibrium one may write
~lia "--[#~ (T) + RgTln {psi(l~).~'iaXia}] "- ].lig = [~ (T) -I- RgTlnpi] (3.41)
leading immediately to
pi = yi e - pO (re) ~'i xi (3.42)
Comparing equations (3.42) and (3.39), it is clear that the only additional