Page 85 - Adsorption Technology & Design, Elsevier (1998)
P. 85
82 Rates of adsorption of gases and vapours by porous media
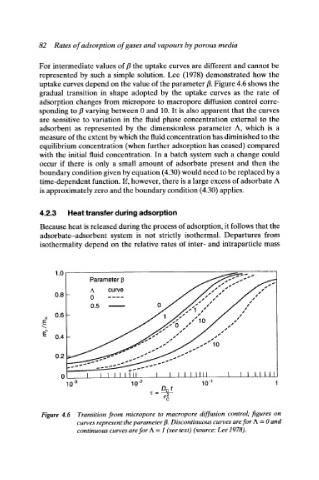
For intermediate values of fl the uptake curves are different and cannot be
represented by such a simple solution. Lee (1978) demonstrated how the
uptake curves depend on the value of the parameter ft. Figure 4.6 shows the
gradual transition in shape adopted by the uptake curves as the rate of
adsorption changes from micropore to macropore diffusion control corre-
sponding to fl varying between 0 and 10. It is also apparent that the curves
are sensitive to variation in the fluid phase concentration external to the
adsorbent as represented by the dimensionless parameter A, which is a
measure of the extent by which the fluid concentration has diminished to the
equilibrium concentration (when further adsorption has ceased) compared
with the initial fluid concentration. In a batch system such a change could
occur if there is only a small amount of adsorbate present and then the
boundary condition given by equation (4.30) would need to be replaced by a
time-dependent function. If, however, there is a large excess of adsorbate A
is approximately zero and the boundary condition (4.30) applies.
4.2.3 Heat transfer during adsorption
Because heat is released during the process of adsorption, it follows that the
adsorbate-adsorbent system is not strictly isothermal. Departures from
isothermality depend on the relative rates of inter- and intraparticle mass
1.0
Parameter 13
A curve
S SS-S S S
0.8 ,==..,= ,=. ,,.
S S S
S S S
i S S
0.5
S
| s S
S
s I
0.6
8 S
s S
S
s 10
E
s
s s
s s
s s
E
s s
0.4
s s
s js
_.. 10
0.2
! I I I I IIII I I I I I IIII I i I I IIII
10. 2 10 "1
10 -3
Dot
1;-- r 2
Figure 4.6 Transition from micropore to macropore diffusion control; figures on
curves represent the parameter ft. Discontinuous curves are for A = 0 and
continuous curves are for A = 1 (see texO (source: Lee 1978).