Page 80 - Adsorption Technology & Design, Elsevier (1998)
P. 80
Rates of adsorption of gases and vapours by porous media 77
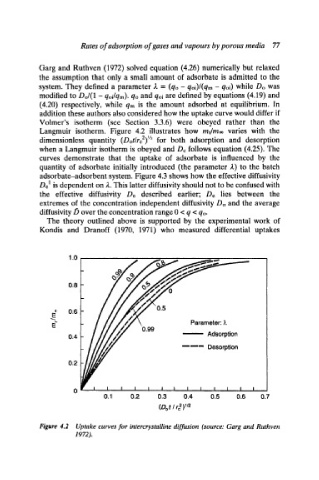
Garg and Ruthven (1972) solved equation (4.26) numerically but relaxed
the assumption that only a small amount of adsorbate is admitted to the
system. They defined a parameter 2 = (qo- qoi)/(qm- qoi) while Do was
modified to Do/(1 -qoi/qm). qo and qoi are defined by equations (4.19) and
(4.20) respectively, while qm is the amount adsorbed at equilibrium. In
addition these authors also considered how the uptake curve would differ if
Volmer's isotherm (see Section 3.3.6) were obeyed rather than the
Langmuir isotherm. Figure 4.2 illustrates how mr~m** varies with the
'
dimensionless quantity (Dot/rc2) /~ for both adsorption and desorption
when a Langmuir isotherm is obeyed and Dc follows equation (4.25). The
curves demonstrate that the uptake of adsorbate is influenced by the
quantity of adsorbate initially introduced (the parameter 2) to the batch
adsorbate-adsorbent system. Figure 4.3 shows how the effective diffusivity
D~ 1 is dependent on A,. This latter diffusivity should not to be confused with
the effective diffusivity D~ described earlier; De lies between the
extremes of the concentration independent diffusivity Do and the average
diffusivity/) over the concentration range 0 < q < qo.
The theory outlined above is supported by the experimental work of
Kondis and Dranoff (1970, 1971) who measured differential uptakes
1.0
0.8
0.5
0.6
Parameter: 7,
0.99
Adsorption
0.4
--"-'- Deso~tion
0.2
0.1 0.2 0.3 0.4 0.5 0.6 0.7
(Dot / r~) v2
Figure 4.2 Uptake curves for intercrystalline diffusion (source: Garg and Ruthven
1972).