Page 128 - Adsorption, Ion Exchange & Catalysis- 2007, Elsevier - Copy
P. 128
Else_AIEC-INGLE_cH003.qxd 7/13/2006 1:45 PM Page 124
124 3. Heterogeneous Processes and Reactor Analysis
35
30 separated chain transition jet
bubbles bubbling regime
25
20
(mm) 15
b
d
10
5 higher orifice
diameter
0
0.0001 0.001 0.01 0.1 1
gas flow rate at orifice opening*orifice diameter
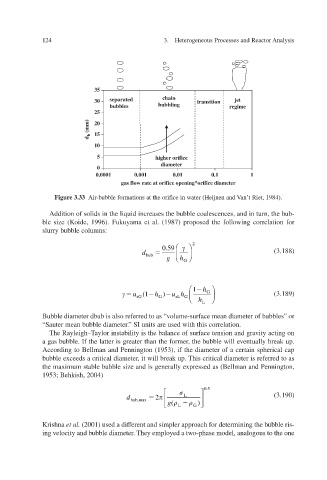
Figure 3.33 Air-bubble formations at the orifice in water (Heijnen and an’t Riet, 1984). V
Addition of solids in the liquid increases the bubble coalescences, and in turn, the b ub-
ble size (K 1996). Fukuyama et al. (1987) proposed the following correlation for
oide,
slurry bubble columns:
0.59 2
d (3.188)
bub
g h G
1 h
h
u (1 ) u h G (3.189)
sG G sL G h L
Bubble diameter dbub is also referred to as “volume-surface mean diameter of bubbles” or
“Sauter mean bubble diameter.” SI units are used with this correlation.
The Rayleigh–Taylor instability is the balance of surface tension and gravity acting on
,
a gas bubble. If the latter is greater than the former the bubble will eentually break up. v
According to Bellman and Pennington (1953), if the diameter of a certain spherical cap
,
bubble exceeds a critical diameter it will break up. This critical diameter is referred to as
the maximum stable bubble size and is generally expressed as (Bellman and Pennington,
1953; Behkish, 2004)
0.5
d 2 L (3.190)
x bub,ma g (
L G )
Krishna et al. (2001) used a different and simpler approach for determining the bubble ris-
ing velocity and bubble diameter. They employed a two-phase model, analogous to the one