Page 168 - Adsorption, Ion Exchange & Catalysis- 2007, Elsevier - Copy
P. 168
Else_AIEC-INGLE_cH003.qxd 7/13/2006 1:45 PM Page 164
164 3. Heterogeneous Processes and Reactor Analysis
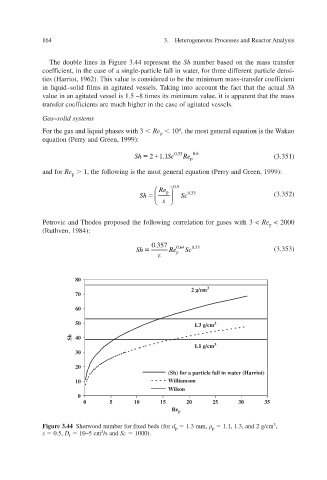
The double lines in Figure 3.44 represent the Sh number based on the mass transfer
,
coefficient, in the case of a single-particle fall in w for three different particle densi-
ater
ties (Harriot, 1962). This value is considered to be the minimum mass-transfer coef icient f
essels.
in liquid–solid films in agitated vTaking into account the fact that the actual Sh
value in an agitated vessel is 1.5 –8 times its minimum v it is apparent that the mass alue,
transfer coefficients are much higher in the case of agitated v essels.
Gas–solid systems
For the gas and liquid phases with 3 Re p 10 4 , the most general equation is the akao W
equation (Perry and Green, 1999):
1.1
2
Sh Sc Re 0.33 p 0.6 (3.351)
and for Re p 1, the following is the most general equation (Perry and Green, 1999):
Re p 0.5
Sh Sc 0.33 (3.352)
Petrovic and Thodos proposed the following correlation for gases with 3 < Re p < 2000
(Ruthven, 1984):
0.357 0.64 0.33
Sh Re Sc p (3.353)
80
2 g/cm 3
70
60
50 1.3 g/cm 3
40 Sh
1.1 g/cm 3
30
20
(Sh) for a particle fall in water (Harriot)
10 Williamson
Wilson
0
0 5 10 15 20 25 30 35
Re p
Figure 3.44 ix Sherwood number for fed beds (for d p 1.3 mm, 1.1, 1.3, and 2 g/cm 3 ,
p
0.5, D f 10–5 cm 2 /s and Sc 1000).