Page 193 - Adsorption, Ion Exchange & Catalysis- 2007, Elsevier - Copy
P. 193
Else_AIEC-INGLE_cH003.qxd 7/13/2006 1:46 PM Page 189
3.8 T Fluid–Solid Fluidized Bed Reactors w o-Phase, 189
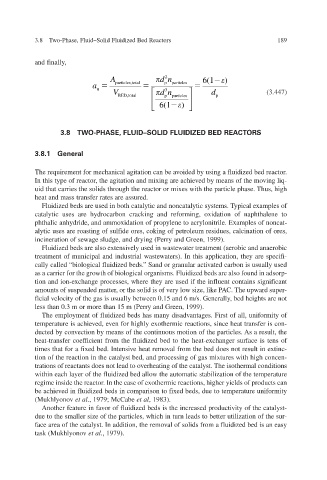
inally and f,
A l particles,tota dn p 2 particles 6(1 )
a
u 3
V dn d (3.447)
BED,tota l p particles p
6(1 6 )
3.8 TWO-PHASE, FLUID–SOLID FLUIDIZED BED REACTORS
3.8.1 General
. v The requirement for mechanical agitation can be aoided by using a fluidized bed reactor
In this type of reactor the agitation and mixing are achieed by means of the moving liq- , v
uid that carries the solids through the reactor or mixes with the particle phase. Thus, high
heat and mass transfer rates are assured.
T Fluidized beds are used in both catalytic and noncatalytic systems. ypical examples of
catalytic uses are hydrocarbon cracking and reforming, oxidation of naphthalene to
phthalic anhydride, and ammoxidation of propylene to acrylonitrile. Examples of noncat-
alytic uses are roasting of sulfide ores, coking of petroleum residues, calcination of ores,
age sludge,
w
incineration of se and drying (Perry and Green, 1999).
Fluidized beds are also extensively used in wastewater treatment (aerobic and anaerobic
w
treatment of municipal and industrial waters). In this application, they are specif i-
aste
” cally called “biological fluidized beds. Sand or granular actiated carbon is usually used v
as a carrier for the growth of biological organisms. Fluidized beds are also found in adsorp-
tion and ion-exchange processes, where they are used if the influent contains signif icant
amounts of suspended matter, or the solid is of vw size, lik C. The upw ery loard super- A e P
ficial velocity of the gas is usually between 0.15 and 6 m/s. Generally, bed heights are not
less than 0.3 m or more than 15 m (Perry and Green, 1999).
The employment of fluidized beds has many disadvantages. First of all, uniformity of
v
v
temperature is achie een for highly exothermic reactions, since heat transfer is con-
ed,
v ducted by conection by means of the continuous motion of the particles. As a result, the
f
heat-transfer coeficient from the fluidized bed to the heat-exchanger surface is tens of
v
times that for a fed bed. Intensie heat remoal from the bed does not result in e xtinc-
ix
v
tion of the reaction in the catalyst bed, and processing of gas mixtures with high concen-
trations of reactants does not lead to overheating of the catalyst. The isothermal conditions
within each layer of the fluidized bed allow the automatic stabilization of the temperature
regime inside the reactor. In the case of exothermic reactions, higher yields of products can
v be achieed in fluidized beds in comparison to fed beds, due to temperature uniformity ix
(Mukhlyonov et al ., 1979; McCabe et al , 1983).
v
a
Another feature in for of fluidized beds is the increased productivity of the catalyst-
due to the smaller size of the particles, which in turn leads to better utilization of the sur-
face area of the catalyst. In addition, the remoal of solids from a fluidized bed is an easy v
task (Mukhlyono v et al. , 1979).