Page 196 - Adsorption, Ion Exchange & Catalysis- 2007, Elsevier - Copy
P. 196
Else_AIEC-INGLE_cH003.qxd 7/13/2006 1:46 PM Page 192
192 3. Heterogeneous Processes and Reactor Analysis
v
of this group hae sizes from 150 µm to 500 µm and densities from 1.4 to 4 g/cm 3 .
Typically used group B materials are glass beads (ballotini) and coarse sand.
• Group C powders: Very fine, cohesi which e po wders are classif , ied into this cate gory v
are incapable of fluidization in the strict sense and tend to rise as a slug of solids. Their
sizes are usually less than 30 µm, and they easily gie rise to channeling. Examples of v
group C materials are talc, flour and starch. ,
• Group D powder s: They are large particles that are distinguished by their ability
to produce deep spouting beds (spurt or jet of gas through the bed). Roasted
coffee beans, lead shot, and some roasted metal ores are examples of group D
materials.
It is noteworthy that the group classification depends not only on the particle but also on
er
,
the gas properties. Moreo the aboe classification is related to the fluidization in the
v
v
presence of air at ambient conditions. For a different fluid and operating conditions, a pow-
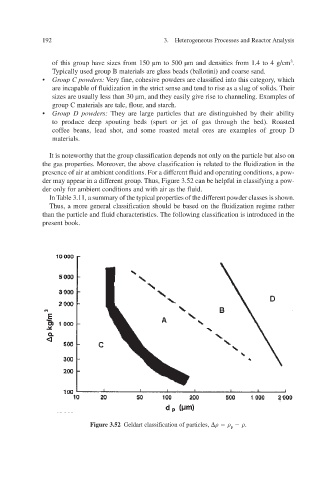
der may appear in a different group. Thus, Figure 3.52 can be helpful in classifying a pow-
der only for ambient conditions and with air as the fluid.
In Table 3.11, a summary of the typical properties of the different powder classes is shown.
Thus, a more general classification should be based on the fluidization regime rather
than the particle and fluid characteristics. The folloication is introduced in the wing classif
present book.
Figure 3.52 Geldart classification of particles, .
p