Page 294 - Adsorption, Ion Exchange & Catalysis- 2007, Elsevier - Copy
P. 294
Else_AIEC-INGLE_cH004.qxd 7/1/2006 6:53 PM Page 290
290 4. Adsorption and Ion Exchange
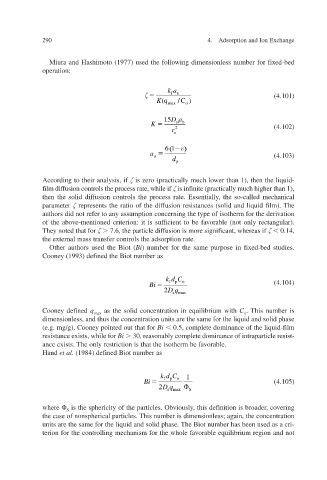
Miura and Hashimoto (1977) used the following dimensionless number for f ed-bed ix
operation:
ka
fu (4.101)
K (q max C )/ o
15 D
K sb
r 2 (4.102)
o
61 )
(
a u (4.103)
d p
According to their analysis, if is zero (practically much lower than 1), then the liquid-
film diffusion controls the process rate, while if is inf ) , inite (practically much higher than 1
,
then the solid diffusion controls the process rate. Essentially the so-called mechanical
parameter represents the ratio of the diffusion resistances (solid and liquid fThe ilm).
authors did not refer to any assumption concerning the type of isotherm for the deri v ation
f
v
of the aboe-mentioned criterion: it is suficient to be forable (not only rectangular). v a
They noted that for 7.6, the particle dif whereas if icant, fusion is more signif 0.14,
the external mass transfer controls the adsorption rate.
Other authors used the Biot ( Bi ) number for the same purpose in fed-bed studies. ix
Cooney (1993) defined the Biot number as
kd C fp o
Bi (4.104)
Dq 2 sma x
ined Cooney def q max as the solid concentration in equilibrium with C . This number is
o
dimensionless, and thus the concentration units are the same for the liquid and solid phase
(e.g. mg/g). Cooney pointed out that for Bi 0.5, complete dominance of the liquid-f ilm
resistance exists, while for Bi 30, reasonably complete dominance of intraparticle resist-
xists. ance eThe only restriction is that the isotherm be f v orable. a
Hand et al . (1984) defined Biot number as
kd C fp o 1
Bi (4.105)
Dq 2 sma x S
where
is the sphericity of the particles. Obviously, this definition is broader, covering
S
the case of nonspherical particles. This number is dimensionless; again, the concentration
units are the same for the liquid and solid phase. The Biot number has been used as a cri-
terion for the controlling mechanism for the whole forable equilibrium region and not v a