Page 299 - Adsorption, Ion Exchange & Catalysis- 2007, Elsevier - Copy
P. 299
Else_AIEC-INGLE_cH004.qxd 7/1/2006 6:53 PM Page 295
4.2 Design of Adsorption and Ion-Exchange Processes 295
Then
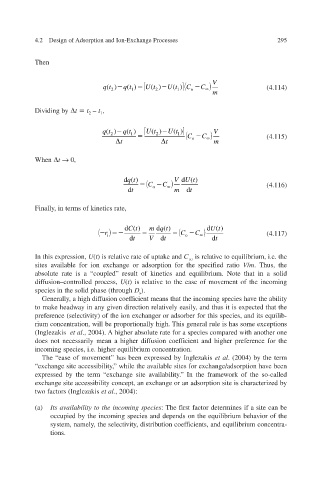
V
qt () 1 U t () U t ( ) C 1 C (4.114)
qt ( )
o
2
2
m
Dividing by t t – t , 1
2
qt () Ut () Ut ( ) V
qt ( )
2 1 2 1 C C (4.115)
t t o m
When t → 0,
d( ) qt V d( ) Ut
C o C (4.116)
d t m d t
Finally, in terms of kinetics rate,
Ct d( ) m qt d( ) Ut d( )
C o C (4.117)
r
i
t d V t d t d
In this e xpression, U ( t ) is relatie rate of uptake and v C is relative to equilibrium, i.e. the
sites aailable for ion exchange or adsorption for the specified ratio V / m . Thus, the
v
absolute rate is a “coupled” result of kinetics and equilibrium. Note that in a solid
v
diffusion–controlled process, U ( t ) is relatie to the ease of moement of the incoming v
species in the solid phase (through D ). s
Generally, a high dificient means that the incoming species hae the ability f fusion coef v
v
,
to make headway in any gien direction relatiely easily and thus it is expected that the
v
preference (selectivity) of the ion exchanger or adsorber for this species, and its equilib-
rium concentration, will be proportionally high. This general rule is has some e xceptions
(Inglezakis et al ., 2004). A higher absolute rate for a species compared with another one
does not necessarily mean a higher diffusion coeficient and higher preference for the
f
incoming species, i.e. higher equilibrium concentration.
The “ease of mo has been expressed by Inglezakis et al . (2004) by the term
v
ement”
“exchange site accessibility while the aailable sites for exchange/adsorption hae been ,” v v
v
ailability
w
expressed by the term “exchange site a In the frameork of the so-called
”
.
exchange site accessibility concept, an exchange or an adsorption site is characterized by
two factors (Inglezakis et al ., 2004):
(a) Its availability to the incoming species : The first factor determines if a site can be
occupied by the incoming species and depends on the equilibrium behavior of the
vity
system, namely the selecti distribution coef and equilibrium concentra- icients, f
,
,
tions.