Page 94 - Adsorption, Ion Exchange & Catalysis- 2007, Elsevier - Copy
P. 94
Else_AIEC-INGLE_cH003.qxd 7/13/2006 1:45 PM Page 90
90 3. Heterogeneous Processes and Reactor Analysis
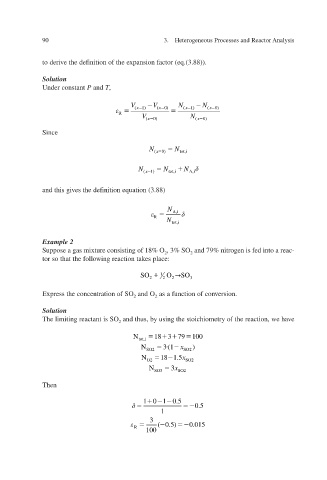
to derive the definition of the expansion factor (eq.(3.88)).
Solution
Under constant P and T ,
V ( x 1) V ( x 0) N ( x 1) N ( x 0)
R
V ( x 0) N ( x 0)
Since
N N
x ( 0) tot,i
N x ( 1) N tot,i N
A,i
v and this gies the definition equation (3.88)
N A,i
R
N
tot,i
Example 2
Suppose a gas mixture consisting of 18% O 2 , 3% SO 2 and 79% nitrogen is fed into a reac-
tor so that the following reaction takes place:
SO O 1 SO
2 2 2 3
Express the concentration of SO 2 and O 2 as a function of con v ersion.
Solution
The limiting reactant is SO 2 and thus, by using the stoichiometry of the reaction, we ha e v
18379
N tot,i 100
1
N 2 SO 3 (
x )
2
SO
18 1.5
N O2 SO2 x
N 3 SO 3 x
2
SO
Then
1 0 1 0.5
0.5
1
3
( 0.5) 0.015
R
100