Page 96 - Adsorption, Ion Exchange & Catalysis- 2007, Elsevier - Copy
P. 96
Else_AIEC-INGLE_cH003.qxd 7/13/2006 1:45 PM Page 92
92 3. Heterogeneous Processes and Reactor Analysis
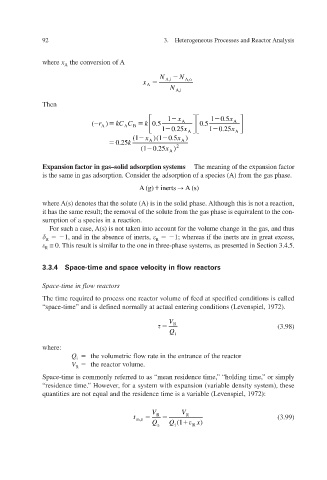
where x A the conversion of A
N A,i N A,o
x
A
N A,i
Then
1 x 1 0.5 x
( r ) C k 0.5 A 0.5 A
C
k
A A B
1 0.25 x A 1 0.25 x A
(1 )( 0.5 x
)
1
x
0.25 k A A
0.25
(1 ) x A 2
Expansion factor in gas–solid adsorption systems The meaning of the expansion factor
is the same in gas adsorption. Consider the adsorption of a species (A) from the gas phase.
A (g) i nert s A (s)
where A(s) denotes that the solute (A) is in the solid phase. Although this is not a reaction,
it has the same result; the removal of the solute from the gas phase is equivalent to the con-
sumption of a species in a reaction.
For such a case, A(s) is not takand thus en into account for the volume change in the gas,
1, and in the absence of inerts, 1; whereas if the inerts are in great e xcess,
R
R
≅ 0. as presented in Section 3.4.5. This result is similar to the one in three-phase systems,
R
3.3.4 Space-time and space velocity in flow reactors
s eactor Space-time in flow r
The time required to process one reactor volume of feed at specified conditions is called
“space-time” and is defined normally at actual entering conditions (Le 1972). v enspiel,
V
R (3.98)
Q
i
where:
Q the volumetric flow rate in the entrance of the reactor
i
V the reactor v olume.
R
”
Space-time is commonly referred to as “mean residence time, “holding time, or simply ”
,
er
“residence time.” Ho for a system with expansion (variable density system), these
we
v
quantities are not equal and the residence time is a v 1972): enspiel, v ariable (Le
V V
t R R (3.99)
m,z
Q z Q i (1 R x )