Page 92 - Adsorption, Ion Exchange & Catalysis- 2007, Elsevier - Copy
P. 92
Else_AIEC-INGLE_cH003.qxd 7/13/2006 1:45 PM Page 88
88 3. Heterogeneous Processes and Reactor Analysis
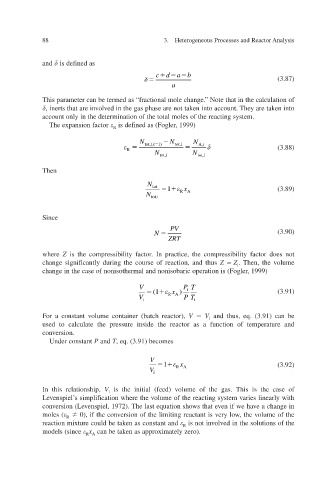
and is defined as
cd a b
(3.87)
a
This parameter can be termed as “fractional mole change. Note that in the calculation of ”
,
inerts that are ined in the gas phase are not taken into account. They are taken into olv v
account only in the determination of the total moles of the reacting system.
The expansion f actor is defined as (F 1999) ogler ,
R
N tot,( 1) x N tot,i N A,i
(3.88)
R
N tot,i N tot,i
Then
N
tot 1 x (3.89)
N tot,i RA
Since
PV
N (3.90)
ZR T
where Z is the compressibility f. In practice, the compressibility factor does not
actor
change significantly during the course of reaction, and thus Z ≈ Z . Then, the volume
i
change in the case of nonisothermal and nonisobaric operation is (F 1999) , ogler
V P T
(1 x ) i (3.91)
V i RA P T i
For a constant volume container (batch reactor), V V and thus, eq. (3.91) can be
i
used to calculate the pressure inside the reactor as a function of temperature and
conversion.
Under constant P and T , eq. (3.91) becomes
V
1 x (3.92)
V i RA
In this relationship, V i is the initial (feed) volume of the gas. This is the case of
Levenspiel’s simplification where the volume of the reacting system varies linearly with
conversion (Levenspiel, 1972). The last equation shows that een if we hae a change in v v
v
moles ( 0), if the conersion of the limiting reactant is very lo the volume of the
w
,
R
reaction mixture could be taken as constant and is not ined in the solutions of the v olv
R
models (since x R A can be taken as approximately zero).