Page 89 - Adsorption, Ion Exchange & Catalysis- 2007, Elsevier - Copy
P. 89
Else_AIEC-INGLE_cH003.qxd 7/13/2006 1:45 PM Page 85
3.3 T Reactors o-Phase Agitated w 85
This kind of reactor is very useful in experimental studies when the goal is the elimina-
tion of the external fluid film resistance. It is used for catalytic as well as adsorption sys-
en, tems (Ruthv 1984).
3.3.3 Material balances in two-phase agitated reactors
Batch reactors
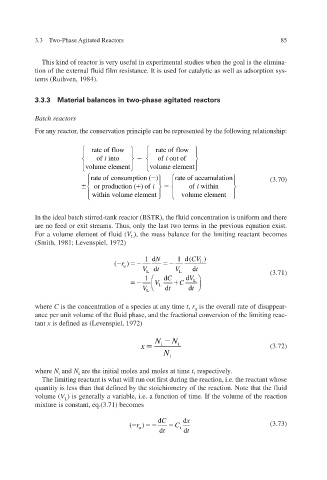
For any reactor, the conservation principle can be represented by the following relationship:
rate of flow rate of flow
of into i of out o i f f
volume e me nt le volume e nt le me
rate of c ( ) rate o accuuo f i n at m l (3.70)
onsumption
or produc tion (() of i of withii n n
within volume e le me nt volume e le nt me
In the ideal batch stirred-tank reactor (BSTR), the fluid concentration is uniform and there
are no feed or exit streams. Thus, only the last two terms in the previous equation e xist.
For a volume element of fluid ( V L ), the mass balance for the limiting reactant becomes
(Smith, 1981; Le 1972) enspiel, v
1d N 1 d( CV )
( r u ) L
V d t V d t
L L (3.71)
1 d C d V L
V L C
V L d t d t
where C is the concentration of a species at any time t , r is the oerall rate of disappear- v
u
ance per unit volume of the fluid phase, and the fractional con v ersion of the limiting reac-
tant x is defined as (Le 1972) enspiel, v
N N
x i t (3.72)
N
i
where N i and N t are the initial moles and moles at time t , respecti . v ely
The limiting reactant is what will run out first during the reaction, i.e. the reactant whose
quantity is less than that defined by the stoichiometry of the reaction. Note that the fluid
volume ( V L ) is generally a v i.e. a function of time. If the volume of the reaction
ariable,
mixture is constant, eq.(3.71) becomes
d C d x
( r u ) C i (3.73)
d t d t