Page 38 - Advanced Gas Turbine Cycles
P. 38
Chapter 2. Reversibility and availability 15
I 1 I
I
I IC"
X I I I 1 Y
I I I
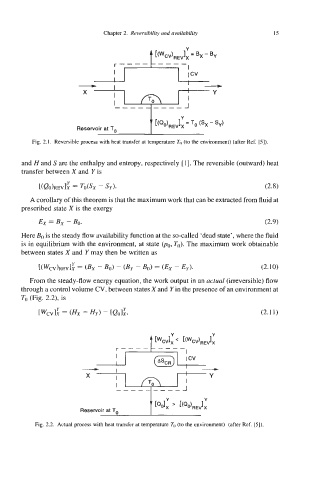
Fig. 2.1. Reversible process with heat transfer at temperature TO (to the environment) (after Ref. [5]).
and Hand S are the enthalpy and entropy, respectively [l]. The reversible (outward) heat
transfer between X and Y is
[<QO>REVli = TdSX - SY). (2.8)
A corollary of this theorem is that the maximum work that can be extracted from fluid at
prescribed state X is the exergy
Ex = Bx - Bo. (2.9)
Here Bo is the steady flow availability function at the so-called 'dead state', where the fluid
is in equilibrium with the environment, at state (Po, To). The maximum work obtainable
between states X and Y may then be written as
[(WCV)REVIi = (BX - BO) - (BY - BO) = (EX - EY). (2.10)
From the steady-flow energy equation, the work output in an actual (irreversible) flow
through a control volume CV, between states X and Y in the presence of an environment at
To (Fig. 2.2), is
Wcvli = (Hx - HY) - [Qoli, (2.1 1)
Reservoir at T,
Fig. 2.2. Actual process with heat transfer at temperature TO (to the environment) (after Ref. [5]).