Page 42 - Advanced Gas Turbine Cycles
P. 42
Chapter 2. Reversibility and availability 19
23. Exergyflux
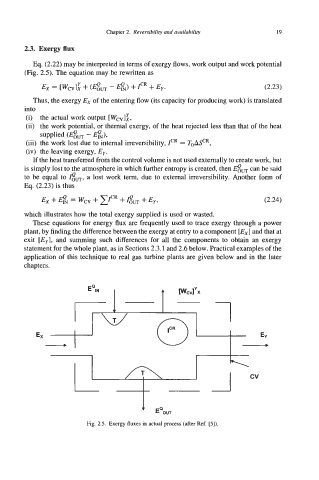
Eq. (2.22) may be interpreted in terms of exergy flows, work output and work potential
(Fig. 2.5). The equation may be rewritten as
Ex = [wcv]: + (Gm - @N) + fR + Eye (2.23)
Thus, the exergy Ex of the entering flow (its capacity for producing work) is translated
into
(i) the actual work output [Wcv]i,
(ii) the work potential, or thermal exergy, of the heat rejected less than that of the heat
supplied (em - @N),
(iii) the work lost due to internal irreversibility, ICR = ToeR,
(iv) the leaving exergy, Ey.
If the heat transferred from the control volume is not used externally to create work, but
is simply lost to the atmosphere in which further entropy is created, then G,,.,. can be said
to be equal to E,,.,., a lost work term, due to external irreversibility. Another form of
Eq. (2.23) is thus
Ex + @N = wcv + XI"" + I& + Ey, (2.24)
which illustrates how the total exergy supplied is used or wasted.
These equations for energy flux are frequently used to trace exergy through a power
plant, by finding the difference between the exergy at entry to a component [Ex] and that at
exit [EY], and summing such differences for all the components to obtain an exergy
statement for the whole plant, as in Sections 2.3.1 and 2.6 below. Practical examples of the
application of this technique to real gas turbine plants are given below and in the later
chapters.
+ E%"T
Fig. 2.5. Exergy fluxes in actual process (after Ref. [5]).