Page 46 - Advanced Gas Turbine Cycles
P. 46
Chapter 2. Reversibility and availability 23
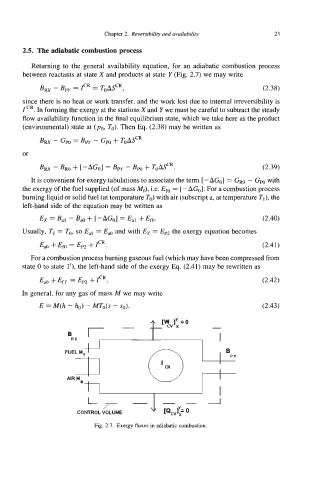
25. The adiabatic combustion process
Returning to the general availability equation, for an adiabatic combustion process
between reactants at state X and products at state Y (Fig. 2.7) we may write
B~ - B~~ = ICR = T~A~C~, (2.38)
since there is no heat or work transfer, and the work lost due to internal irreversibility is
I CR. In forming the exergy at the stations X and Y we must be careful to subtract the steady
flow availability function in the final equilibrium state, which we take here as the product
(environmental) state at (pol TO). Then Eq. (2.38) may be written as
B~ - G~ = B~~ - G~ + T,A~C~
or
B, - BRO + [-AGO] = Bpy - BW + TOAsCR. (2.39)
It is convenient for exergy tabulations to associate the term [-AGO] = so Gpo with
-
the exergy of the fuel supplied (of mass Mf), i.e. Em = [-AGO]. For a combustion process
burning liquid or solid fuel (at temperature To) with air (subscript a, at temperature TI), the
left-hand side of the equation may be written as
Ex = B,I - B,o + [-AGO] = Ea] + Efo. (2.40
Usually, TI = To, so E,, = Eno and with E, = Em the exergy equation becomes
E,~ +E~ E~ + ICR. (2.41)
=
For a combustion process burning gaseous fuel (which may have been compressed from
state 0 to state 1’), the left-hand side of the exergy Eq. (2.41) may be rewritten as
Ed + Efl, = Em + ICR. (2.42)
In general, for any gas of mass M we may write
E = M(h - kO) - MTO(S - SO), (2.43)
COFlTROL 5- -.1K$T
VOLUME
Fig. 2.7. Exergy fluxes in adiabatic combustion.