Page 40 - Advanced Gas Turbine Cycles
P. 40
Chapter 2. Reversibility and availability 17
The maximum (reversible) work obtained from the 'inner' control volume CV is
therefore equal to
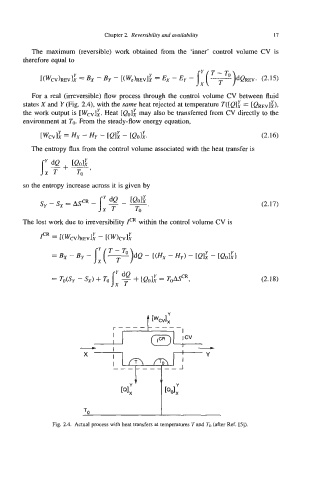
For a real (irreversible) flow process through the control volume CV between fluid
states X and Y (Fig. 2.4), with the sum heat rejected at temperature T([Q]i = [Q,,]:),
the work output is [Wcv];. Heat [eo]: may also be transferred from CV directly to the
environment at TO. From the steady-flow energy equation,
The entropy flux from the control volume associated with the heat transfer is
-+-, [Qol!
dQ
TO
so the entropy increase across it is given by
(2.17)
The lost work due to irreversibility within the control volume CV is
fR = r(wcv)REvl; - Kwkvl;
T-To
= Bx - BY - Ix (7)dQ - (WX HY) - [el: - [Qol:}
-
(2.18)
TO
Fig. 2.4. Actual process with heat transfers at temperatures T and To (after Ref. [5]).