Page 85 - Advanced Gas Turbine Cycles
P. 85
Chapter 4. Cycle eficiency with turbine cooling (cwlingJow rates specified) 61
4.3.2. The simple approach
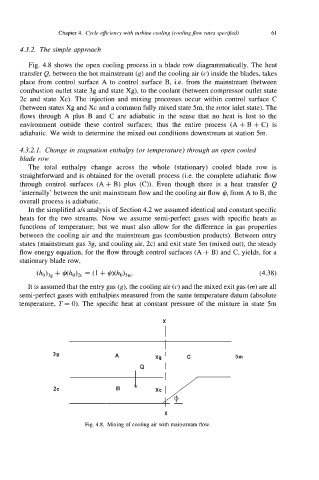
Fig. 4.8 shows the open cooling process in a blade row diagrammatically. The heat
transfer Q, between the hot mainstream (g) and the cooling air (c) inside the blades, takes
place from control surface A to control surface B, i.e. from the mainstream (between
combustion outlet state 3g and state Xg), to the coolant (between compressor outlet state
2c and state Xc). The injection and mixing processes occur within control surface C
(between states Xg and Xc and a common fully mixed state 5m, the rotor inlet state). The
flows through A plus B and C are adiabatic in the sense that no heat is lost to the
environment outside these control surfaces; thus the entire process (A + B + C) is
adiabatic. We wish to determine the mixed out conditions downstream at station 5m.
4.3.2. I. Change in stagnation enthalpy (or temperature) through an open cooled
blade row
The total enthalpy change across the whole (stationary) cooled blade row is
straightforward and is obtained for the overall process (i.e. the complete adiabatic flow
through control surfaces (A+B) plus (C)). Even though there is a heat transfer Q
‘internally’ between the unit mainstream flow and the cooling air flow $, from A to B, the
overall process is adiabatic.
In the simplified a/s analysis of Section 4.2 we assumed identical and constant specific
heats for the two streams. Now we assume semi-perfect gases with specific heats as
functions of temperature; but we must also allow for the difference in gas properties
between the cooling air and the mainstream gas (combustion products). Between entry
states (mainstream gas 3g, and cooling air, 2c) and exit state 5m (mixed out), the steady
flow energy equation, for the flow through control surfaces (A + B) and C, yields, for a
stationary blade row,
(ho)3g + 4@0)2c = (1 + $)(ho)Sm. (4.38)
It is assumed that the entry gas (g), the cooling air (c) and the mixed exit gas (m) are all
semi-perfect gases with enthalpies measured from the same temperature datum (absolute
temperature, T = 0). The specific heat at constant pressure of the mixture in state 5m
x
I ~~
39 A x9 I C 5m
Q I
B .1
2c
X
Fig. 4.8. Mixing of cooling air with mainstream flow.