Page 102 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 102
this analysis indicates that the overall barrier results from compensating trends in the four 81
components. These results pertain to a fixed geometry and do not take into account bond
angle and bond length adjustments in response to rotation. TOPIC 1.2
Heteroatom
Hyperconjugation
(Anomeric Effect) in
CH 3 OH Acyclic Molecules
CH 3 CH 3 CH 3 NH 2
−1 423 −0 766 −0 440
E Lewis
+4 953 +2 920 +1 467
E deloc
−0 827 −0 488 −1 287
E steric
+2 009 +1 483 +0 475
E 2×2
+4 712 +3 149 +0 215
E total
The methanol rotational barrier was further explored, using the approach described
above for ethane. 109 The effect of changes in molecular structure that accompany
rotation were included. The approach taken was to systematically compare the effect on
the rotational barrier of each specific interaction, e.g., hyperconjugation and exchange
repulsion, and to determine the effect on molecular geometry, i.e., bond lengths and
angles. The analysis of electrostatic forces (nuclear-nuclear, electron-electron, and
nuclear-electron) showed that it was the nuclear-electron forces that are most important
in favoring the staggered conformation, whereas the other two actually favor the
eclipsed conformation. The structural response to the eclipsed conformation is to
lengthen the C−O bond, destabilizing the molecule. The more favorable nuclear-
electron interaction in the staggered conformation is primarily a manifestation of
hyperconjugation. In comparison with ethane, a major difference is the number of
hyperconjugative interactions. The oxygen atom does not have any antibonding orbitals
associated with its unshared electron pairs and these orbitals cannot act as acceptors.
The oxygen unshared electrons function only as donors to the adjacent anti C–H bonds
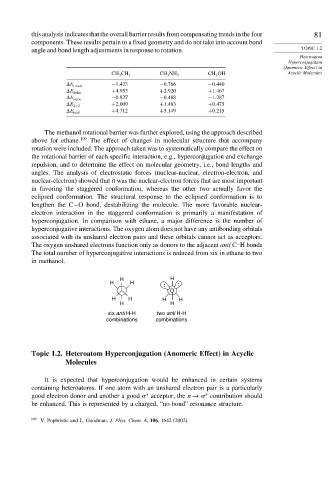
The total number of hyperconjugative interactions is reduced from six in ethane to two
in methanol.
H H
H H
O
H H H H
H H
six anti H-H two anti H-H
combinations combinations
Topic 1.2. Heteroatom Hyperconjugation (Anomeric Effect) in Acyclic
Molecules
It is expected that hyperconjugation would be enhanced in certain systems
containing heteroatoms. If one atom with an unshared electron pair is a particularly
good electron donor and another a good acceptor, the n → contribution should
∗
∗
be enhanced. This is represented by a charged, “no-bond” resonance structure.
109
V. Pophristic and L. Goodman, J. Phys. Chem. A, 106, 1642 (2002).