Page 107 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 107
86 to the greater s character in the carbon orbital. Proponents of VB description argue that
the properties of cyclopropane are well described by the bent bond idea and that no
CHAPTER 1 121
other special characteristics are needed to explain bonding in cyclopropane. There is,
Chemical Bonding however, an unresolved issue. Cyclopropane has a total strain energy of 27.5 kcal/mol.
and Molecular Structure
This is only slightly greater than that for cyclobutane (26.5 kcal/mol), which suggests
that there might be some special stabilizing feature present in cyclopropane.
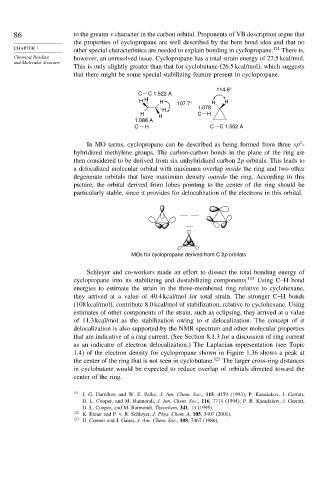
114.8°
C C 1.522 A
H H H 107.7° H H
H 1.078
H H C H
1.088 A
C H C C 1.502 A
2
In MO terms, cyclopropane can be described as being formed from three sp -
hybridized methylene groups. The carbon-carbon bonds in the plane of the ring are
then considered to be derived from six unhybridized carbon 2p orbitals. This leads to
a delocalized molecular orbital with maximum overlap inside the ring and two other
degenerate orbitals that have maximum density outside the ring. According to this
picture, the orbital derived from lobes pointing to the center of the ring should be
particularly stable, since it provides for delocalization of the electrons in this orbital.
MOs for cyclopropane derived from C 2p orbitals
Schleyer and co-workers made an effort to dissect the total bonding energy of
cyclopropane into its stabilizing and destabilizing components. 122 Using C–H bond
energies to estimate the strain in the three-membered ring relative to cyclohexane,
they arrived at a value of 40.4 kcal/mol for total strain. The stronger C–H bonds
(108 kcal/mol), contribute 8.0 kcal/mol of stabilization, relative to cyclohexane. Using
estimates of other components of the strain, such as eclipsing, they arrived at a value
of 11.3 kcal/mol as the stabilization owing to delocalization. The concept of
delocalization is also supported by the NMR spectrum and other molecular properties
that are indicative of a ring current. (See Section 8.1.3 for a discussion of ring current
as an indicator of electron delocalization.) The Laplacian representation (see Topic
1.4) of the electron density for cyclopropane shown in Figure 1.36 shows a peak at
the center of the ring that is not seen in cyclobutane. 123 The larger cross-ring distances
in cyclobutane would be expected to reduce overlap of orbitals directed toward the
center of the ring.
121
J. G. Hamilton and W. E. Palke, J. Am. Chem. Soc., 115, 4159 (1993); P. Karadakov, J. Gerratt,
D. L. Cooper, and M. Raimondi, J. Am. Chem. Soc., 116, 7714 (1994); P. B. Karadakov, J. Gerratt,
D. L. Cooper, and M. Raimondi, Theochem, 341, 13 (1995).
122 K. Exner and P. v. R. Schleyer, J. Phys. Chem. A, 105, 3407 (2001).
123
D. Cremer and J. Gauss, J. Am. Chem. Soc., 108, 7467 (1986).