Page 110 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 110
X 2 X X X 89
+ +
TOPIC 1.3
X X
X Bonding in
Cyclopropane and Other
Small Ring Compounds
X+ X
X +
+
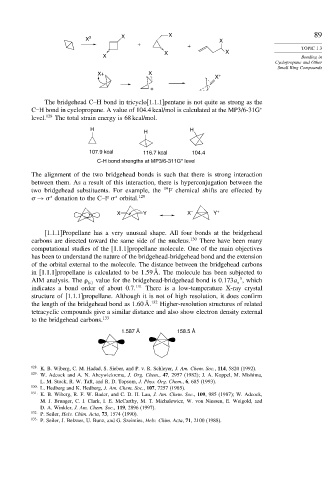
The bridgehead C–H bond in tricyclo[1.1.1]pentane is not quite as strong as the
C–H bond in cyclopropane. A value of 104.4 kcal/mol is calculated at the MP3/6-31G ∗
level. 128 The total strain energy is 68 kcal/mol.
H H
H
107.9 kcal 116.7 kcal 104.4
C-H bond strengths at MP3/6-311G* level
The alignment of the two bridgehead bonds is such that there is strong interaction
between them. As a result of this interaction, there is hyperconjugation between the
two bridgehead substituents. For example, the 19 F chemical shifts are effected by
→ donation to the C–F orbital. 129
∗
∗
X Y X – Y +
[1.1.1]Propellane has a very unusual shape. All four bonds at the bridgehead
carbons are directed toward the same side of the nucleus. 130 There have been many
computational studies of the [1.1.1]propellane molecule. One of the main objectives
has been to understand the nature of the bridgehead-bridgehead bond and the extension
of the orbital external to the molecule. The distance between the bridgehead carbons
in [1.1.1]propellane is calculated to be 1 59Å. The molecule has been subjected to
3
AIM analysis. The c value for the bridgehead-bridgehead bond is 0 173a , which
indicates a bond order of about 0.7. 131 There is a low-temperature X-ray crystal
structure of [1.1.1]propellane. Although it is not of high resolution, it does confirm
the length of the bridgehead bond as 1 60Å. 132 Higher-resolution structures of related
tetracyclic compounds give a similar distance and also show electron density external
to the bridgehead carbons. 133
1.587 Å 158.5 Å
128
K. B. Wiberg, C. M. Hadad, S. Sieber, and P. v. R. Schleyer, J. Am. Chem. Soc., 114, 5820 (1992).
129 W. Adcock and A. N. Abeywickrema, J. Org. Chem., 47, 2957 (1982); J. A. Koppel, M. Mishima,
L. M. Stock, R. W. Taft, and R. D. Topsom, J. Phys. Org. Chem., 6, 685 (1993).
130 L. Hedberg and K. Hedberg, J. Am. Chem. Soc., 107, 7257 (1985).
131
K. B. Wiberg, R. F. W. Bader, and C. D. H. Lau, J. Am. Chem. Soc., 109, 985 (1987); W. Adcock,
M. J. Brunger, C. I. Clark, I. E. McCarthy, M. T. Michalewicz, W. von Niessen, E. Weigold, and
D. A. Winkler, J. Am. Chem. Soc., 119, 2896 (1997).
132 P. Seiler, Helv. Chim. Acta, 73, 1574 (1990).
133
P. Seiler, J. Belzner, U. Bunz, and G. Szeimies, Helv. Chim. Acta, 71, 2100 (1988).