Page 112 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 112
91
TOPIC 1.3
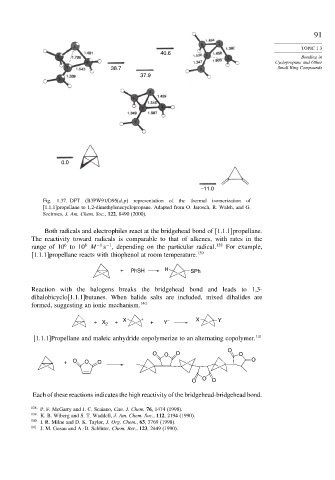
40.6
Bonding in
Cyclopropane and Other
38.7 Small Ring Compounds
37.9
0.0
–11.0
Fig. 1.37. DFT (B3PW91/D95(d,p) representation of the thermal isomerization of
[1.1.1]propellane to 1,2-dimethylenecyclopropane. Adapted from O. Jarosch, R. Walsh, and G.
Szeimies, J. Am. Chem. Soc., 122, 8490 (2000).
Both radicals and electrophiles react at the bridgehead bond of [1.1.1]propellane.
The reactivity toward radicals is comparable to that of alkenes, with rates in the
6
8
range of 10 to 10 M −1 −1 138 For example,
s , depending on the particular radical.
[1.1.1]propellane reacts with thiophenol at room temperature. 139
+ PhSH H SPh
Reaction with the halogens breaks the bridgehead bond and leads to 1,3-
dihalobicyclo[1.1.1]butanes. When halide salts are included, mixed dihalides are
formed, suggesting an ionic mechanism. 140
+ X + + Y – X Y
+ X 2
[1.1.1]Propellane and maleic anhydride copolymerize to an alternating copolymer. 141
O
O O O O
+ O O O O
O O O
Each of these reactions indicates the high reactivity of the bridgehead-bridgehead bond.
138
P. F. McGarry and J. C. Scaiano, Can. J. Chem. 76, 1474 (1998).
139
K. B. Wiberg and S. T. Waddell, J. Am. Chem. Soc., 112, 2194 (1990).
140 I. R. Milne and D. K. Taylor, J. Org. Chem., 63, 3769 (1998).
141
J. M. Gosau and A.-D. Schlüter, Chem. Ber., 123, 2449 (1990).