Page 117 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 117
96
CHAPTER 1
Chemical Bonding +1
and Molecular Structure IP .
E
0
.
. –1
EA .
N
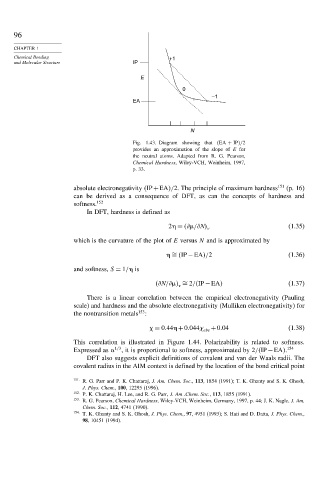
Fig. 1.43. Diagram showing that EA + IP /2
provides an approximation of the slope of E for
the neutral atoms. Adapted from R. G. Pearson,
Chemical Hardness, Wiley-VCH, Weinheim, 1997,
p. 33.
absolute electronegativity IP+EA /2. The principle of maximum hardness 151 (p. 16)
can be derived as a consequence of DFT, as can the concepts of hardness and
softness. 152
In DFT, hardness is defined as
2 = *%/*N (1.35)
which is the curvature of the plot of E versus N and is approximated by
IP −EA /2 (1.36)
and softness, S = 1/ is
*N/*%
2/ IP −EA (1.37)
There is a linear correlation between the empirical electronegativity (Pauling
scale) and hardness and the absolute electronegativity (Mulliken electronegativity) for
the nontransition metals 153 :
= 0 44 +0 044 abs +0 04 (1.38)
This correlation is illustrated in Figure 1.44. Polarizability is related to softness.
Expressed as 1/3 , it is proportional to softness, approximated by 2/ IP −EA . 154
DFT also suggests explicit definitions of covalent and van der Waals radii. The
covalent radius in the AIM context is defined by the location of the bond critical point
151
R. G. Parr and P. K. Chattaraj, J. Am. Chem. Soc., 113, 1854 (1991); T. K. Ghanty and S. K. Ghosh,
J. Phys. Chem., 100, 12295 (1996).
152 P. K. Chattaraj, H. Lee, and R. G. Parr, J. Am .Chem. Soc., 113, 1855 (1991).
153 R. G. Pearson, Chemical Hardness, Wiley-VCH, Weinheim, Germany, 1997, p. 44; J. K. Nagle, J. Am.
Chem. Soc., 112, 4741 (1990).
154
T. K. Ghanty and S. K. Ghosh, J. Phys. Chem., 97, 4951 (1993); S. Hati and D. Datta, J. Phys. Chem.,
98, 10451 (1994).