Page 115 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 115
94
CHAPTER 1
Chemical Bonding
and Molecular Structure
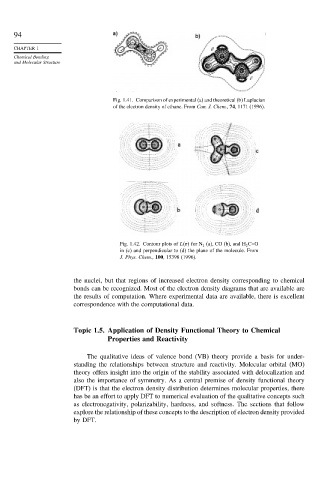
Fig. 1.41. Comparison of experimental (a) and theoretical (b) Laplacian
of the electron density of ethane. From Can. J. Chem., 74, 1171 (1996).
Fig. 1.42. Contour plots of L r for N 2 (a), CO (b), and H 2 C=O
in (c) and perpendicular to (d) the plane of the molecule. From
J. Phys. Chem., 100, 15398 (1996).
the nuclei, but that regions of increased electron density corresponding to chemical
bonds can be recognized. Most of the electron density diagrams that are available are
the results of computation. Where experimental data are available, there is excellent
correspondence with the computational data.
Topic 1.5. Application of Density Functional Theory to Chemical
Properties and Reactivity
The qualitative ideas of valence bond (VB) theory provide a basis for under-
standing the relationships between structure and reactivity. Molecular orbital (MO)
theory offers insight into the origin of the stability associated with delocalization and
also the importance of symmetry. As a central premise of density functional theory
(DFT) is that the electron density distribution determines molecular properties, there
has be an effort to apply DFT to numerical evaluation of the qualitative concepts such
as electronegativity, polarizability, hardness, and softness. The sections that follow
explore the relationship of these concepts to the description of electron density provided
by DFT.