Page 114 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 114
93
TOPIC 1.4
Representation of
Electron Density by the
Laplacian Function
Fig. 1.39. Contour maps of L r for: (a) ethane in a
plane bisecting anti-hydrogens; (b) ethene in the molecular
plane; (c) ethene perpendicular to the molecular plane, and
(d) ethyne. The solid lines depict the zero-flux surfaces of the
C and H atoms. From J. Phys. Chem. 100, 15398 (1996).
addition to the C–H bonds, the contours indicate the electron density associated with
the unshared pairs on oxygen. Figure 1.42d, shows L perpendicular to the plane of the
molecule and is influenced by the component of the C=O bond. It shows greater
electron density around oxygen, which is consistent with the expectation that carbon
would have a partial positive change.
These diagrams can help to visualize the electron density associated with these
prototypical molecules. We see that most electron density is closely associated with
2
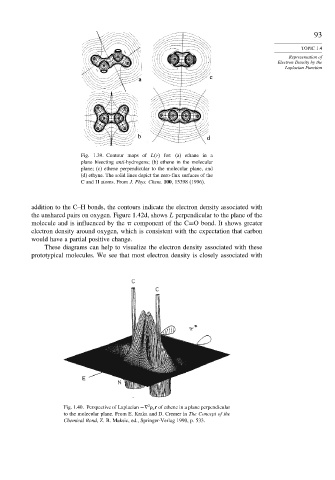
Fig. 1.40. Perspective of Laplacian −! c r of ethene in a plane perpendicular
to the molecular plane. From E. Kraka and D. Cremer in The Concept of the
Chemical Bond, Z. B. Maksic, ed., Springer-Verlag 1990, p. 533.