Page 118 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 118
97
TOPIC 1.5
Application of Density
Functional Theory
to Chemical Properties
and Reactivity
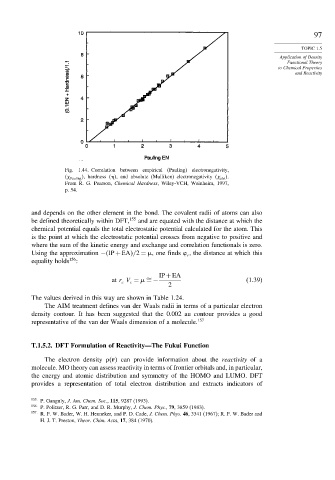
Fig. 1.44. Correlation between empirical (Pauling) electronegativity,
Pauling , hardness , and absolute (Mulliken) electronegativity abs .
From R. G. Pearson, Chemical Hardness, Wiley-VCH, Weinheim, 1997,
p. 54.
and depends on the other element in the bond. The covalent radii of atoms can also
be defined theoretically within DFT, 155 and are equated with the distance at which the
chemical potential equals the total electrostatic potential calculated for the atom. This
is the point at which the electrostatic potential crosses from negative to positive and
where the sum of the kinetic energy and exchange and correlation functionals is zero.
Using the approximation − IP +EA /2 = %, one finds , the distance at which this
r
equality holds 156 :
IP +EA
at r V = $
− (1.39)
c
c
2
The values derived in this way are shown in Table 1.24.
The AIM treatment defines van der Waals radii in terms of a particular electron
density contour. It has been suggested that the 0.002 au contour provides a good
representative of the van der Waals dimension of a molecule. 157
T.1.5.2. DFT Formulation of Reactivity—The Fukui Function
The electron density r can provide information about the reactivity of a
molecule. MO theory can assess reactivity in terms of frontier orbitals and, in particular,
the energy and atomic distribution and symmetry of the HOMO and LUMO. DFT
provides a representation of total electron distribution and extracts indicators of
155 P. Ganguly, J. Am. Chem. Soc., 115, 9287 (1993).
156 P. Politzer, R. G. Parr, and D. R. Murphy, J. Chem. Phys., 79, 3859 (1983).
157
R. F. W. Bader, W. H. Henneker, and P. D. Cade, J. Chem. Phys. 46, 3341 (1967); R. F. W. Bader and
H. J. T. Preston, Theor. Chim. Acta, 17, 384 (1970).